Porphyrins and similar compounds have been intensively studied, both to study their functions in natural systems and to use their chemical properties. Corrole, for example, has applications in catalysis, sensors, and medicine.
The controlled aggregation of the corrole macrocycles is a key step for the preparation of functional corrole-based materials. In the porphyrin family, one of the most studied macrocycles is the commercially available 5,10,10,20-tetrasulfonatophenylporphyrin, which in acidic aqueous solutions spontaneously forms ordered aggregates. Unfortunately, the synthetic strategy used for the sulfonation of porphyrins cannot be applied to the corrole ring.
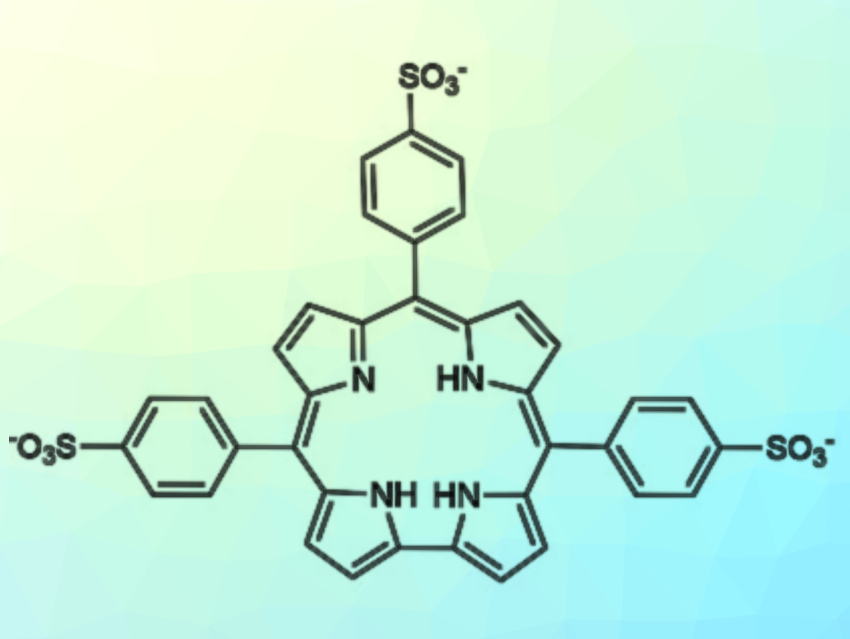
Roberto Paolesse, University of Rome “Tor Vergata”, Italy, and colleagues have developed two synthetic routes to obtain the water-soluble 5,10,15-tris(4-sulfonatophenyl)corrole (pictured). The first approach uses a chlorosulfonation of the phosphorus complex of 5,10,15‐tris(4‐trimethylsilylphenyl)corrole. It provides only low yields of the desired compound in the form of its phosphorus complex. The second approach uses a reaction between pyrrole and 4‐chlorosulfonyl benzaldehyde, followed by hydrolysis of the obtained chlorosulfonyl-substituted corrole, to give the desired product in 10 % yield.
Different from the analogous porphyrin, this water-soluble corrole does not aggregate in acidic solutions. Instead, it is present as a monomeric zwitterionic species even at pH = 2. This result may be attributed to the lower symmetry of the corrole ring, which reduces the efficiency of molecular packing. However, the lack of aggregation could allow the use of this corrole for the preparation of hybrid nanostructured systems, where this macrocycle is conjugated with positively charged porphyrinoids. In future work, the researchers want to prepare these hybrid aggregates and explore their performance as sensing materials.
- 5,10,15-Tris(4-sulfonatophenyl)corrole Synthesis,
Fabrizio Caroleo, Sara Nardis, Greta Petrella, Martina Bischetti, Daniel O. Cicero, Damiano Genovese, Liviana Mummolo, Luca Prodi, Rosalba Randazzo, Alessandro D’Urso, Roberto Paolesse,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201901155