Porous solid Brønsted acids could be useful as catalysts for many challenging and important reactions. Metal–organic frameworks (MOFs) can be used for this type of catalytic reaction. However, MOFs with chiral Brønsted acid sites are rare, which makes it difficult to achieve high enantioselectivities.
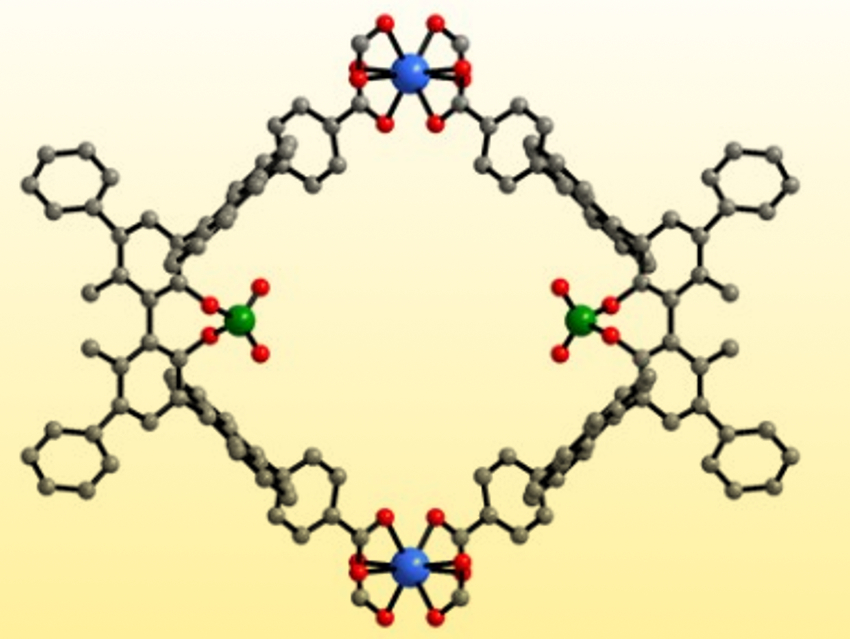
Yan Liu, Yong Cui, Shanghai Jiao Tong University, China, Jianwen Jiang, National University of Singapore, and colleagues have introduced catalytically active phosphoric acids into indium-based MOFs. The team synthesized three chiral Brønsted-acidic MOFs (pictured below). The MOFs were prepared from indium nitrate and enantiopure tetracarboxylate linkers (H4Ln) that are derived from 1,1′-biphenolphosphoric acid. The carboxylate groups coordinate the indium ions to generate extended 3D networks. The bulky ligands protect the phosphoric acid groups and prevent them from coordinating. As a result, the free phosphoric acid groups are periodically arranged inside the MOFs’ pores.

The resulting confined, strong Brønsted acids catalyze both asymmetric condensation/amine addition reactions and asymmetric imine reduction reactions. Stereoselectivities can be tuned by modifying the biphenol substituents. MOF 1 generally provides the best results. Density functional theory (DFT) calculations suggest that the porous framework stabilizes intermediates and transition states, leading to high enantioselectivities.
- Chiral Phosphoric Acids in Metal-Organic Frameworks with Enhanced Acidity and Tunable Catalytic Selectivity,
Xu Chen, Hong Jiang, Xu Li, Bang Hou, Wei Gong, Xiaowei Wu, Xing Han, Fanfan Zheng, Yan Liu, Jianwen Jiang, Yong Cui,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201908959