Cyanides are commonly used ligands in transition-metal chemistry. In general, the CN– ion can bind through its carbon atom or its nitrogen atom. It is also sometimes encountered as a bridging ligand. While the more covalent CN compounds of the late main-group elements can be either cyanides (C-bound) or isocyanides (N-bound), alkali metal cyanides are ionic with no preferred directionality.
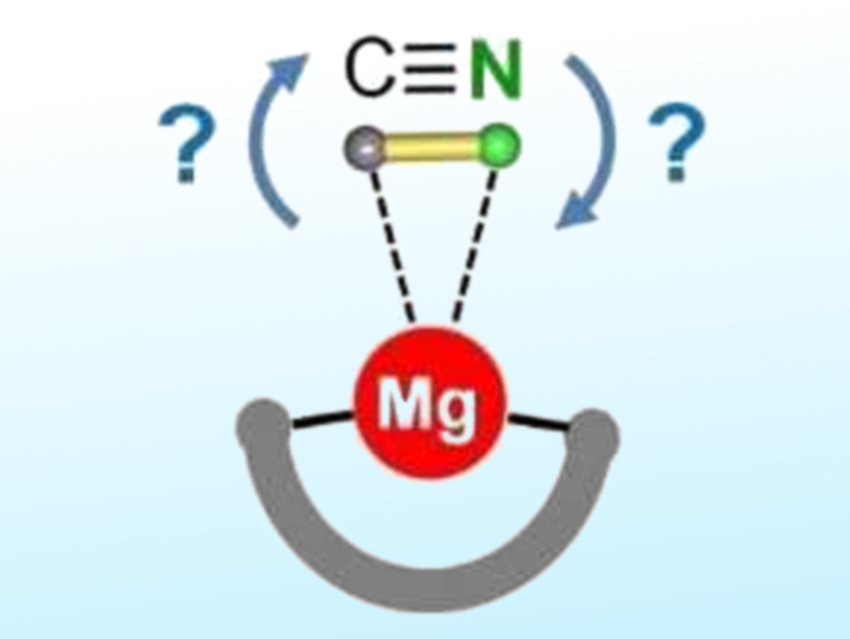
Sjoerd Harder and colleagues, University of Erlangen-Nürnberg, Germany, have investigated the cyanide/isocyanide preference for the less ionic magnesium complexes. The team synthesized a magnesium complex with a bulky dipyrromethene ligand that encapsulates the metal (pictured schematically) and prevents the formation of Mg-CN-Mg bridges. They observed a strong preference for the Mg-NC isocyanide isomer (95:5 ratio). This was determined by crystal structure determinations and density-functional (DFT) calculations.
This result may be explained by the HSAB (“hard and soft acids and bases”) concept: The hard Mg2+ ion prefers interaction with the harder nitrogen site. Isomerization occurs fairly easily, but NMR studies show that this process can be stopped by cooling to –85 °C. The preference for Mg-NC bonding is important, e.g., when discussing mechanisms for magnesium-catalyzed cyanation reactions. In future work, the researchers hope to isolate monomeric heavier alkaline-earth-metal cyanides, which might feature an unusual side-on coordination mode for cyanide.
- Magnesium Cyanide or Isocyanide?,
Gerd Ballmann, Holger Elsen, Sjoerd Harder,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201909511




Good