Cross-linking reactions of DNA with proteins are important for the synthesis of stable bioconjugates, but also for capturing and identifying proteins binding to certain DNA sequences. However, the synthesis of DNA with reactive groups is challenging. The known functional groups typically react with cystein. Cross-linking to lysine has been achieved through the reductive amination of aldehyde-modified DNA, but this reaction requires the use of toxic NaBH3CN as an additional reducing agent.
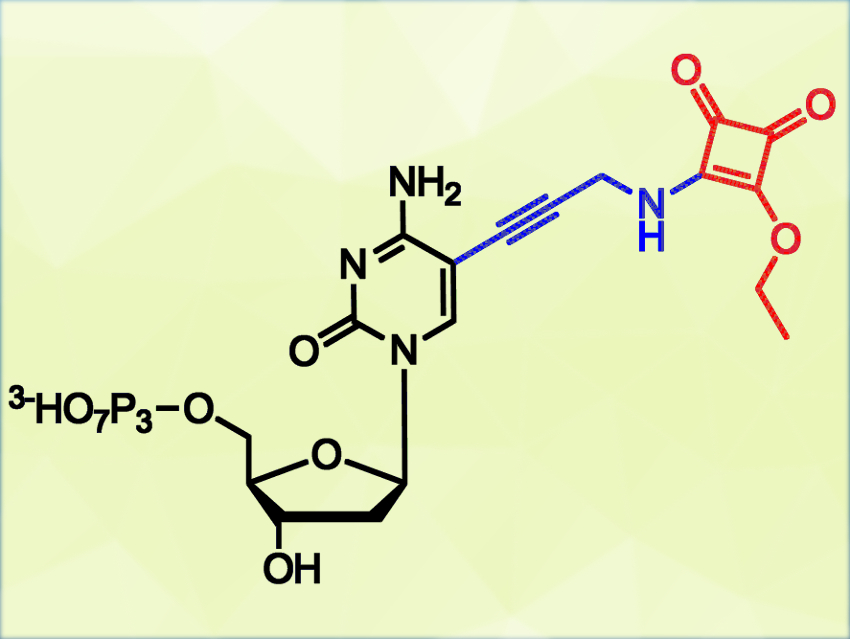
Michal Hocek, Charles University and Czech Academy of Sciences, both Prague, Czech Republic, and colleagues have synthesized 2′-deoxycytidine triphosphates with a squaramate group (pictured in red) attached to the nucleobase. These nucleotides can be used as substrates for DNA polymerases in the enzymatic synthesis of reactively labeled DNA. The squaramate groups in the resulting DNA probes can react with lysine-containing peptides. The probes can also form cross-links with histone proteins, which have a strong affinity to DNA.
The reaction proceeds under physiological conditions (at pH 7.4–9) and does not require additional reagents. It has possible applications in bioconjugations and chemical biology.
- Squaramate-Modified Nucleotides and DNA for Specific Cross-Linking with Lysine-Containing Peptides and Proteins,
Ivana Ivancová, Radek Pohl, Martin Hubálek, Michal Hocek,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201906737