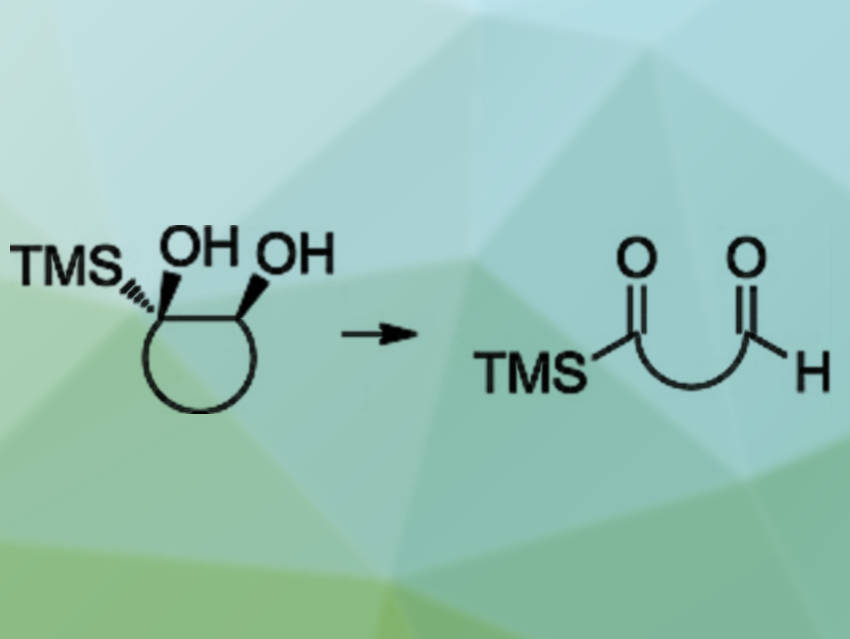
Acylsilanes are a useful compound class in organic chemistry with versatile reactivity. Commonly, acylsilanes are synthesized either by the umpolung of an aldehyde and a subsequent nucleophilic substitution at a silyl halide or by the nucleophilic attack of a silyl anion on a carbonyl compound.
Peter Metz and colleagues, Technische Universität Dresden, Germany, have developed an alternative method for the preparation of acylsilanes. The team cleaved 1,2-dihydroxysilanes with sodium periodate (NaIO4) as an oxidant to give bifunctional acylsilanes (pictured). The required substrates can be synthesized from ketones via conversion to tosylhydrazones, followed by a Shapiro reaction to give a vinylsilane, and a final cis-dihydroxylation to give the desired 1,2-dihydroxysilanes.
The reaction proceeds under mild conditions and gives good to excellent yields of the desired acylsilanes. In some cases, the products can be easily obtained in high purity after a simple aqueous workup. The team also tested a single acyclic 1,2-dihydroxysilane substrate, which was readily cleaved, as well.
- Preparation of Functionalized Acylsilanes by Diol Cleavage of Cyclic 1,2-Dihydroxysilanes,
Patrick Zimdars, Kristin Böhlig, Peter Metz,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900726