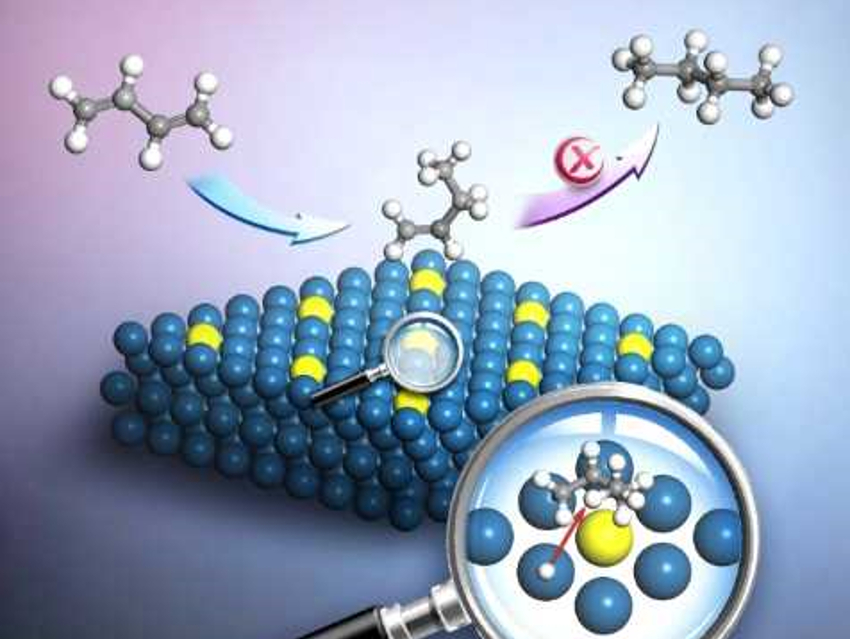
Combining platinum with other metals is an effective way to tune its catalytic performance. However, the rational design of bimetallic catalysts to tailor their selectivity is challenging.
Chaoquan Hu, Chinese Academy of Sciences, Beijing, Qingshan Zhu, University of the Chinese Academy of Sciences, Beijing, Ding Ma, Peking University, Beijing, China, and colleagues have developed a process to rationally design platinum-based alloys that can inhibit the over-hydrogenation of butadiene. Butadiene hydrogenation on Pt particles has four products: 1‐butene and trans‐/cis‐2‐butene from partial hydrogenation, and n‐butane from complete hydrogenation. Alloying Pt with another metal can significantly change the product distribution.
The team developed a model consisting of isolated single heteroatoms embedded into platinum (pictured), which they used for rational screening using density functional theory (DFT) calculations. They found that the diffusion of H atoms adsorbed on the platinum (111) surface towards the C=C bond is a key step for controlling over-hydrogenation. A second hydrogenation can be inhibited by alloying Pt with Ag, Au, Zn, and Sn. These metals increase the diffusion barrier for hydrogen atoms.
The team tested several platinum-based catalysts—pure Pt and PtM (M=Ru, Zn, Ag)—and found that the theoretical results were consistent with experimental observations. The researchers are now studying hydrogen migration as a function of several reaction conditions, such as pressure and temperature, for further catalyst design.
- Hydrogen diffusion guided design of Pt-based alloys for inhibition of butadiene over-hydrogenation,
Chaoquan Hu, Ding Ma, Jiahan Sun, Dongjie Wang, Mingyuan Shao, Qingshan Zhu, Mi Peng,
ChemPhysChem 2019.
https://doi.org/10.1002/cphc.201900380