The enzyme glucose-6-phosphate dehydrogenase (G6PD) catalyzes the first step of the pentose phosphate pathway (PPP). PPP is a metabolic pathway that is important for the production of nucleotides and fatty acids. The G6PD-catalyzed step forms the reducing agent NADPH, a coenzyme important for keeping cells healthy and alive. However, G6PD is only active in its dimeric or tetrameric form, and mutations can weaken these structures, leading to inactivation.
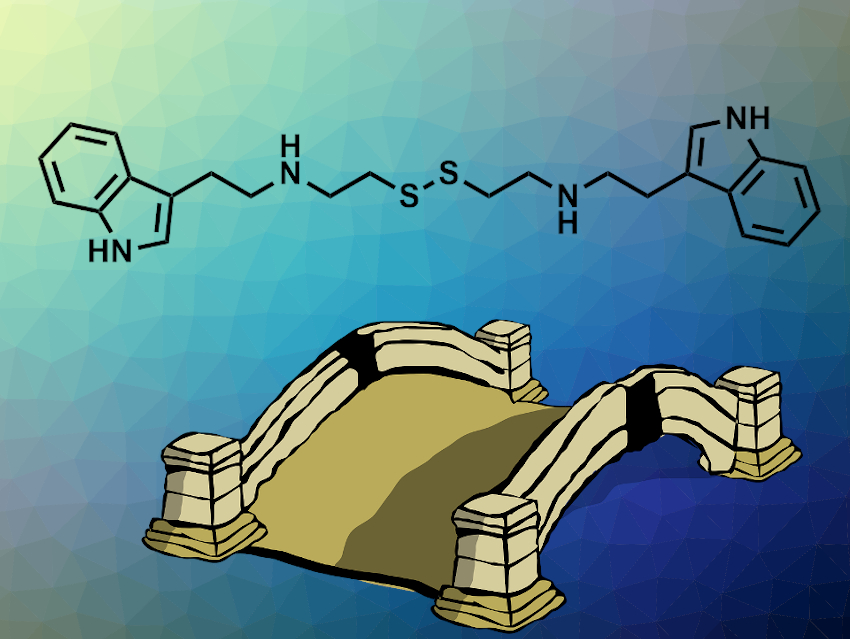
Daria Mochly-Rosen, Stanford University School of Medicine, CA, USA, and colleagues have identified a small-molecule activator that promotes the dimer formation of G6PD. In a biochemical screening, the team found that AG1 (pictured) activated several pathogenic forms of G6PD. Their biochemical studies suggest that the activation is based on a bridging effect of AG1 within the dimeric structure. The structure of the linker was varied, and it was found that the disulfide is not crucial for the activating effect and can be replaced with simple alkyl chains.
According to the researchers, the activation by small molecules could help develop a therapy for G6PD deficiency, which is a common human enzyme-related disease. The strategy could be applied to other protein–protein interactions (PPIs) as well.
- Small‐Molecule Activators of Glucose‐6‐phosphate Dehydrogenase (G6PD) Bridging the Dimer Interface,
Andrew G. Raub, Sunhee Hwang, Naoki Horikoshi, Anna D. Cunningham, Simin Rahighi, Soichi Wakatsuki, Daria Mochly‐Rosen,
ChemMedChem 2019, 14, 1321–1324.
https://doi.org/10.1002/cmdc.201900341