Malaria is a parasitic disease transferred by mosquitos. The species of Plasmodium falciparum is responsible for most malaria cases in Africa, and infections often lead to death. Drug resistance adds to the need for new antimalarial drugs.
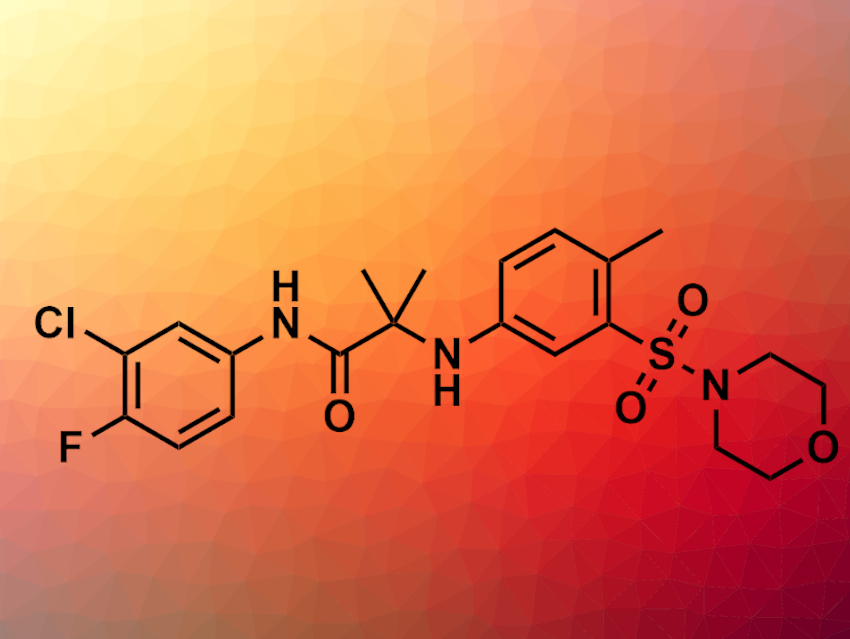
Ian H. Gilbert, Kevin D. Read, both University of Dundee, UK, and colleagues, synthesized a library of 31 compounds and evaluated their antimalarial potency. As a starting point, the researchers selected an amidoacetamide from a high-throughput screening by GlaxoSmithKline for further optimization. The chosen compound showed promising antimalarial activity against P. falciparum, and was not cytotoxic for mammalian cells at concentrations tested. Modification of the linker led to a compound (pictured) with antimalarial potency that was improved more than 10-fold compared with the lead structure. It selectively inhibited P. falciparum with a half maximal effective concentration (EC50) of 7 nM.
However, the metabolic stability and solubility of the compound were still poor, and could not be improved while maintaining the antimalarial activity. According to the researchers, it is nevertheless a valuable compound for the identification of the molecular target and the search for new antimalarial drugs.
- Substituted Aminoacetamides as Novel Leads for Malaria Treatment,
Neil R. Norcross, Caroline Wilson, Beatriz Baragaña, Irene Hallyburton, Maria Osuna-Cabello, Suzanne Norval, Jennifer Riley, Daniel Fletcher, Robert Sinden, Michael Delves, Andrea Ruecker, Sandra Duffy, Stephan Meister, Yevgeniya Antonova-Koch, Benigno Crespo, Cristina de Cózar, Laura M. Sanz, Francisco Javier Gamo, Vicky M. Avery, Julie A. Frearson, David W. Gray, Alan H. Fairlamb, Elizabeth A. Winzeler, David Waterson, Simon F. Campbell, Paul A. Willis, Kevin D. Read, Ian H. Gilbert,
ChemMedChem 2019, 14, 1329–1335.
https://doi.org/10.1002/cmdc.201900329