Shedding Light on Organic Versatility
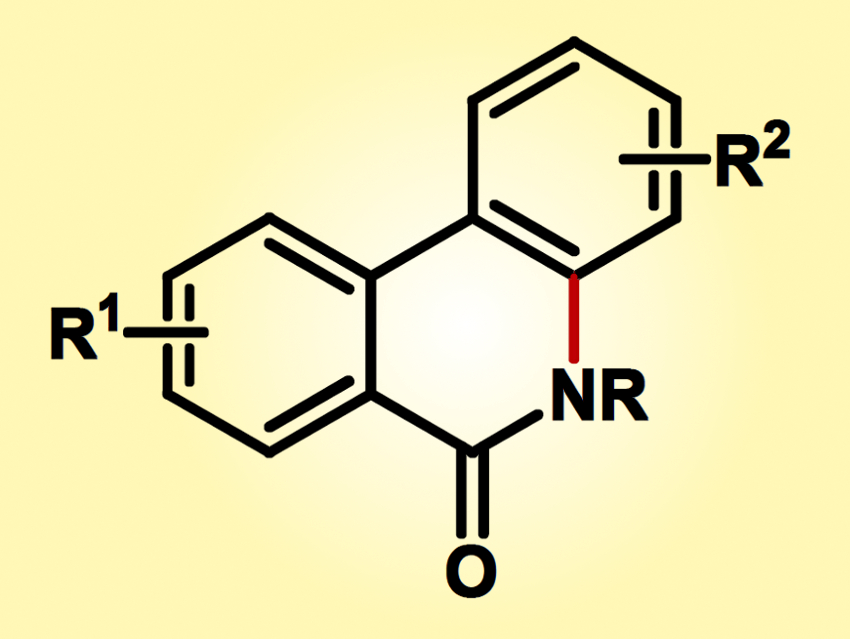
Phenanthridinones (pictured) are versatile organic molecules that can be readily manipulated, functionalized, and otherwise converted. The tricyclic aromatic heterocyclic amide motif that is found in this class of compounds is present in many natural products and has biological activity. As such, it is a useful starting point for generating biologically active molecules.
Intramolecular amidation is often used to construct the main backbone of the molecule, the lactam group, and then a costly metal catalyst is required to complete the preparation of the phenanthridinone.
Homing in on Amidation
Research teams have homed in on the essential amidation of the carbon–hydrogen bond to make phenanthridinones with varying degrees of success. Copper iodide catalysts and even hypervalent iodine reagents have proven useful, but with various limitations. In one case, for example, it was only possible to make N-alkoxy and N-phthaloyl compounds [1]. In 2018, an intramolecular C–H bond amidation of biphenyl using an expensive iridium-containing photocatalyst was reported [2].
Akichika Itoh and colleagues, Gifu Pharmaceutical University, Japan, have taken some inspiration from this earlier work and especially the ability to drive a conversion using light, albeit at raised temperatures and with a metal catalyst. Their success in the catalyzed radical imidation of a heteroaromatic ring [3] led them to the current work on the synthesis of phenanthridinones.
The team’s method wholly avoids the need for a precious metal catalyst and involves a visible-light-driven oxidative amidation of the requisite C–H bond. The room-temperature visible-light irradiation of N-aryl biphenylcarboxamide, 1-chloroanthraquinone (1-Cl-AQN) catalyst, and potassium carbonate in chloroform solvent triggers a radical cyclization that generates the phenanthridinone. The inexpensive commercial 1-Cl-AQN organophotocatalyst the team used represents a step change from the costly precious metal catalysts that have until now seemed necessary for such conversions. They also point out that their reaction proceeds under very mild conditions; no heating is required, neither are strong acids or bases.
The researchers add that in their reaction, they have successfully used nitrogen compounds substituted with electron-deficient aromatic rings and heterocycles as the substrates for the conversion. This is a first in a phenanthridinone synthesis under photoredox conditions. A very respectable yield of 35 % was achieved initially for the reaction. Lower yields were attained in polar solvents, hence the use of chloroform. Ultimately, tuning the system with only a very small amount of photosensitizer, the 1-Cl-AQN and 0.1 equivalents of potassium carbonate, the team reached an excellent yield of 96 %.
Scope and Limitations
The next step was to investigate the generality of the optimized reaction. Might it be extended to related compounds and what are its limits? Preliminary tests to ascertain the scope of the reaction involved using different substrates with various substituents on the nitrogen atom of the carboxamide group. If there was an attached electron-rich aryl group, then yields were excellent, while halogen substituents led to good yields. The products of reactions of those kinds of compound could then be further functionalized. Bulky substituents did not seem to hinder the reaction.
The team found that radical scavengers, such as 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) hindered the reaction entirely. This is what one would assume with a mechanism involving a free radical pathway. Indeed, the presence of a radical scavenger lends support to the team’s promised radical mechanism. The fact that nothing happens in the dark adds to this, as the radical generator is light-activated. Moreover, if the reaction is attempted under argon it also fails, suggesting that oxygen is needed too.
“We have reported a transition-metal-free phenanthridinone synthesis using an organophotocatalyst,” the team states, “This is the first method of phenanthridinone synthesis that progresses via a direct C–H amidation without using a transition metal under visible-light irradiation, and the reaction proceeds under mild conditions at low cost.” The team concludes, “this [reaction] has expanded the applicability of the substrates to a greater extent than previous photooxidative methods.”
- Transition-Metal-Free Synthesis of Phenanthridinones through Visible-Light-Driven Oxidative C–H Amidation,
Kaoru Usami, Eiji Yamaguchi, Norihiro Tada, Akichika Itoh,
Eur. J. Org. Chem. 2019.
https://doi.org/10.1002/ejoc.201900536
References
- [1] Intramolecular Cyclization with Nitrenium Ions Generated by Treatment ofN-Acylaminophthalimides with Hypervalent Iodine Compounds: Formation of Lactams and Spiro-Fused Lactams,
Yasuo Kikugawa, Akira Nagashima, Takeshi Sakamoto, Etsuko Miyazawa, Megumi Shiiya,
J. Org. Chem. 2003, 68, 6739–6744.
https://doi.org/10.1021/jo0347009 - [2] Visible-Light-Photocatalyzed Synthesis of Phenanthridinones and Quinolinones via Direct Oxidative C–H Amidation,
Yonghoon Moon, Eunyoung Jang, Soyeon Choi, Sungwoo Hong,
Org. Lett. 2017, 20, 240–243.
https://doi.org/10.1021/acs.orglett.7b03600 - [3] Cross-Dehydrogenative C–H Amination of Indoles under Aerobic Photo-oxidative Conditions,
Tomoaki Yamaguchi, Eiji Yamaguchi, Akichika Itoh,
Org. Lett. 2017, 19, 1282–1285.
https://doi.org/10.1021/acs.orglett.7b00026


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
