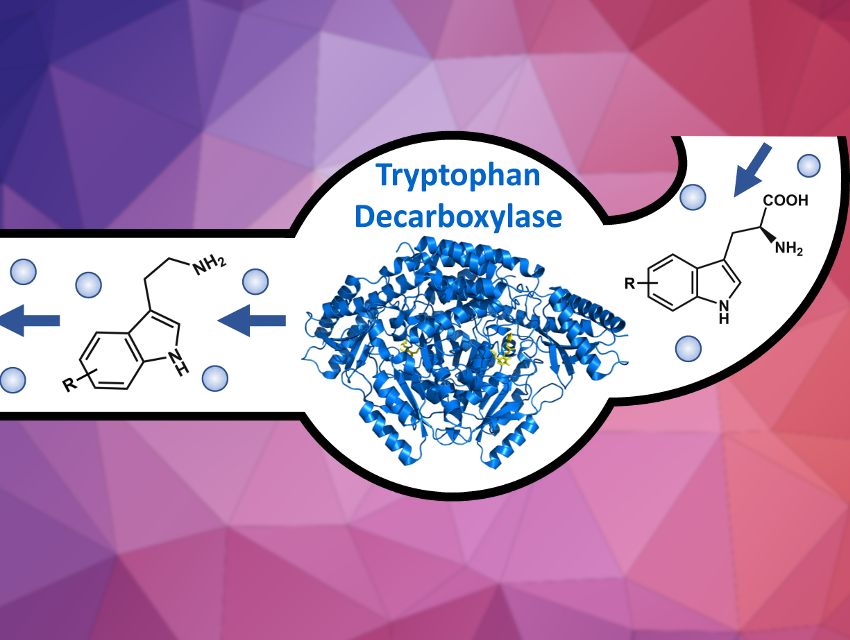
Tryptamines are common building blocks for indole alkaloid natural products, a valuable class of clinically used drugs. However, the synthesis of alkaloid derivatives is often hindered by their structural complexity. Biocatalytic access to tryptamine derivatives could provide improved synthetic routes to possible drug candidates.
Andrew Richard Buller and colleagues, University of Wisconsin – Madison, USA, have developed a simple synthetic procedure to make tryptamine analogs using enzyme catalysis. This strategy uses the rich chemical diversity of commercially available indoles. A tryptophan synthase enzyme was engineered to convert simple indoles into the corresponding tryptophan analogs. The tryptophan derivatives still needed to be converted to tryptamines. However, no decarboxylase enzyme had been previously reported that can convert a range of tryptophans to the desired tryptamines.
The team found a highly proficient tryptophan decarboxylase in the gut microbe Ruminococcus gnavus, which was used to produce over 20 different tryptamines. The tryptophan synthase and decarboxylase enzymes can be combined in a one-pot reaction to produce these products under mild, aqueous conditions. The team hopes to use these enzymes to enable access to complex natural product derivatives.
- Facile in vitro biocatalytic production of diverse tryptamines,
Allwin McDonald, Lydia Perkins, Andrew Richard Buller,
ChemBioChem 2019.
https://doi.org/10.1002/cbic.201900069