The development of water-oxidation electrocatalysts (WOEs) is important for various types of electrochemical devices for energy conversion or chemical transformations. However, it is still challenging to achieve high efficiency and high durability for WOEs used in acidic media.
Younan Xia, Georgia Institute of Technology and Emory University, Atlanta, USA, and colleagues have developed a simple synthesis of iridium-based cubic nanocages as WOE electrocatalysts. The team first synthesized Pd@Ir core–shell nanocubes via the deposition of Ir atoms on Pd nanocubes and then selectively removed the Pd cores by chemical etching.
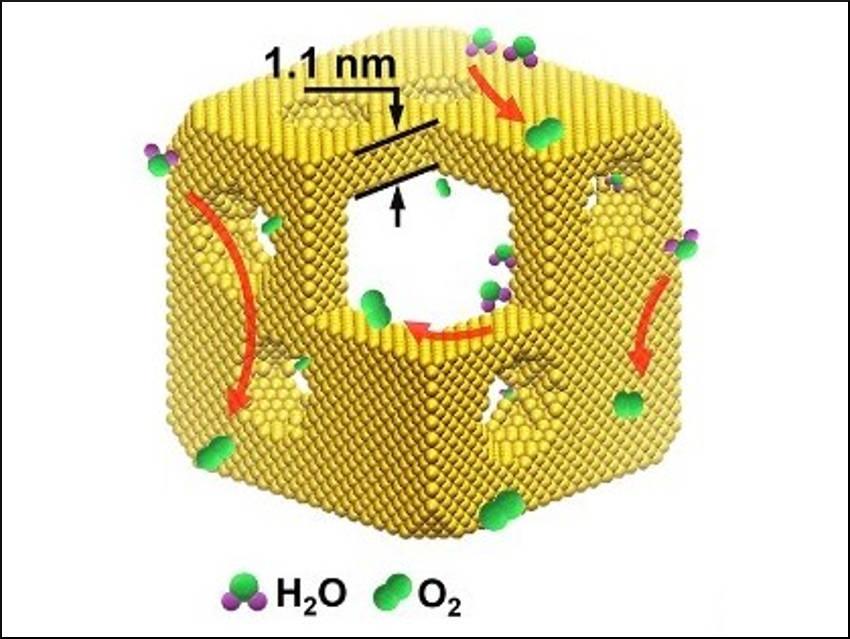
The nanocages (pictured) have a composition of Ir44Pd10, well-defined {100} facets, and porous walls that are only 1.1 nm thick. The catalysts have a substantially enhanced activity and durability; they outperform most of the existing WOEs for acidic media.
According to the researchers, the improved performance is caused by the highly efficient utilization of the iridium atoms and by the open structure, which promotes the electrochemical oxidation of Ir to active IrOx. The work could help with the design and synthesis of cost-effective electrocatalysts with high activity without compromising their durability.
- Iridium-Based Cubic Nanocages with 1.1-nm-Thick Walls: A Highly Efficient and Durable Electrocatalyst for Water Oxidation in an Acidic Medium,
Jiawei Zhu, Zitao Chen, Minghao Xie, Zhiheng Lyu, Miaofang Chi, Manos Mavrikakis, Wanqin Jin, Younan Xia,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201901732