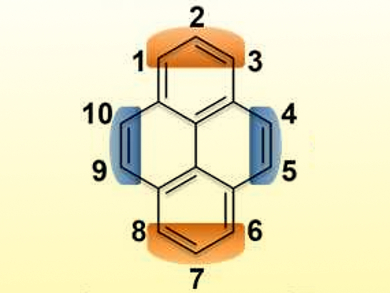
Polycyclic aromatic compounds based on pyrene (pictured) are often useful chromophores and can also serve as semiconductors in (opto-)electronic devices. The extension of the so-called K-Region of pyrene (pictured in blue) has provided various π-conjugated systems with useful properties. However, the non-K-region annulation of pyrene (region pictured in orange) has been very rare due to the low reactivity at the 2- and 7-positions.
Xiao-Ye Wang, Klaus Müllen, and colleagues, Max Planck Institute for Polymer Research, Mainz, Germany, have found that a direct C–H borylation can readily occur at the 2- and 7-positions of pyrene. The team started from di- or tetrabromopyrene derivatives, depending on whether a single-side or double-side annelated compound was the goal. Then they used a Suzuki coupling to introduce two or four 2‐methoxyphenyl groups. Finally, a demethylation and the direct C−H borylation using BBr3 were performed. The reaction gives oxaborin‐annelated pyrene derivatives (examples pictured below) in high yields.
This approach provides a path to a new kind of non-K-region-annelated pyrene-based chromophores. For example, a strong blue fluorescence (λmax: 442 nm) was observed for the single-side annulated molecule 1 (pictured below). The double-side annelated compound with alkyl substituents 2b (pictured below) has a bright green fluorescence (λmax: 525 nm) and a strong self-assembly tendency. The synthesized pyrene derivatives are promising for photonic and electronic applications.

- Direct C−H Borylation at the 2- and 2,7-Positions of Pyrene Leading to Brightly Blue- and Green-Emitting Chromophores,
Xuelin Yao, Ke Zhang, Klaus Müllen, Xiao-Ye Wang,
Asian J. Org. Chem. 2018.
https://doi.org/10.1002/ajoc.201800501The article is part of an Asian Journal of Organic Chemistry Special Issue on Organic Materials.