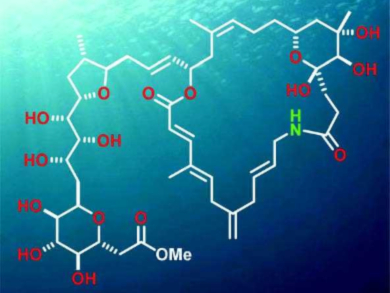
Marine dinoflagellates are simple plankton organisms, yet many of them produce remarkably complex metabolites. A recent example of these compounds is belizentrin (pictured), which is structurally rather unique but has proved too unstable for comprehensive biological profiling. All that could be shown was that belizentrin affects neuronal networks at nanomolar concentration.
Alois Fürstner and colleagues, Max Planck Institute for Coal Research, Mülheim, Germany, have achieved the first total synthesis of belizentrin methyl ester. The structure consists of a hydrophilic tail and a polyunsaturated macrocyclic core. The synthetic route features a catalytic asymmetric hetero-Diels–Alder reaction, several elaborate cross-coupling steps, an unusual ylide formation, and a rare example of a macrocyclization by aminolysis of a lactone precursor. Because of the base-sensitivity of the product, the final fragment coupling—which attached the polyol side chain to the macrocycle—proved exceedingly difficult; a modification of the Kocienski olefination had to be devised to reach the final product.
The team deliberately targeted the methyl ester of belizentrin for stability reasons. The 13C NMR spectrum of belizentrin methyl ester shows a systematic drift relative to the literature data of parent belizentrin, but shows a reasonable to excellent match after a correction for this difference. The 1H NMR data are in good accord with the reported spectra of belizentrin.
- Total Synthesis of Belizentrin Methyl Ester: Report on a Likely Conquest,
Felix Anderl, Sylvester Größl, Conny Wirtz, Alois Fürstner,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201805125