Electrochemical double-layer capacitors (EDLCs) based on symmetric carbon electrodes have a high power output and are long-lived. However, they have a low energy density compared to other power sources. Pore morphology optimization and the modification of carbon materials with pseudocapacitive materials can improve the electrodes’ capacitance, but at the expense of conductivity, cycling stability, and volumetric energy density.
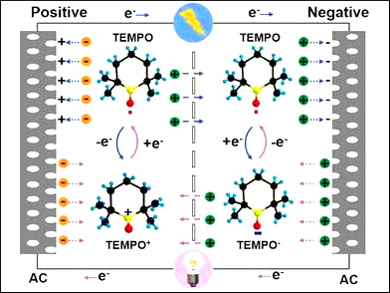
Huiqiao Li, Huazhong University of Science and Technology, Wuhan, China, Yonggang Wang, Fudan University, Shanghai, China, and colleagues have added the ambipolar organic radical 2,2,6,6‐tetramethylpiperidinyloxyl (TEMPO) into the electrolyte of EDLCs to enhance their energy density. This strategy results in additional pseudocapacitance, i.e., the improved storage of electricity in an electrochemical capacitor, by a simultaneous oxidization/reduction at the positive/negative electrodes. The redox reaction occurs at the electrode/electrolyte interface (pictured).
This strategy successfully improves the energy density with little effect on the cycling stability. The presence of the radical does not increase the device volume. A high energy density of 51 Wh kg–1 was obtained, 2.4 times that of an analogous capacitor without TEMPO, together with a long life of 4,000 cycles.
- Electrochemical Double-Layer Capacitor Energized by Adding an Ambipolar Organic Redox Radical into the Electrolyte,
Lintong Hu, Chao Shi, Kai Guo, Tianyou Zhai, Huiqiao Li, Yonggang Wang,
Angew. Chem. Int. Ed. 2018, 57, 8214–8218.
https://doi.org/10.1002/anie.201804582