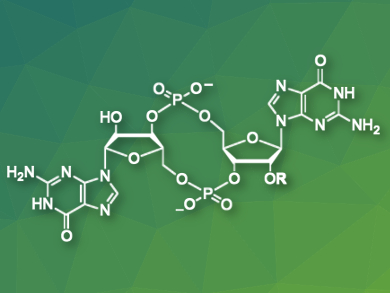
The emergence of multidrug-resistant bacteria has made it important to search for new strategies to combat bacterial infections. Cyclic diguanylate (c-di-GMP), an intracellular signaling molecule, is a promising target for such strategies because it regulates important cellular processes such as biofilm formation. Defensive biofilms shield bacteria from conventional antibiotics and make infections more difficult to treat.
Helma Wennemers, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have developed peptides that target and bind c-di-GMP selectively. Proline-rich tetrapeptides with cationic and aromatic groups were identified in a combinatorial library screening and used to bind c-di-GMP in an aqueous solution. Binding studies suggest that π–π-stacking between a naphthyl group of the peptide and the guanine moieties of c-di-GMP, as well as electrostatic interactions and/or hydrogen bonding, are responsible for the tight and selective binding.
The newly developed tetrapeptides inhibit the biofilm formation of Pseudomonas aeruginosa, which is considered a critical drug-resistant pathogen by the World Health Organization (WHO). The researchers claim that the peptides do not kill bacteria at the low doses required for biofilm inhibition, and thus, avoid promoting bacterial resistance.
- Functionalized Proline-Rich Peptides Bind the Bacterial Second Messenger c-di-GMP,
Helma Wennemers, Carlotta Foletti, Rolf Kramer, Harald Mauser, Urs Jenal, Konrad Bleicher,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201801845