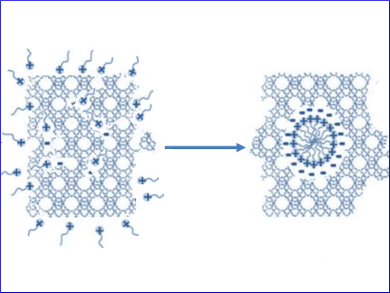
Zeolites are the most-used catalysts in oil refining and petrochemistry operations. Their selectivity has been improved and the throughput and quality of the product have been increased by introducing tailored mesoporosity into the zeolites. Mesopore formation in zeolites can be achieved by either surfactant-templating or by dissolution and reassembly. Despite the commercial use of surfactant-templating, the driving forces responsible for the pore formation in this process are still unknown.
Javier Garcia-Martinez and colleagues, University de Alicante, Spain, have studied the kinetics of the development of intracrystalline mesoporosity in zeolites for the first time. They used a combination of in situ techniques such as synchrotron X-ray diffraction (XRD) with ex-situ gas adsorption and thermogravimetric analysis/differential thermal analysis (TG/DTA). The team determined the apparent activation energies of the different processes involved in the mesostructuring of USY zeolites, an ultra-stable form of zeolite Y.
The results provide insights into the mechanism of the surfactant-templating method (pictured), which opens up new possibilities for the introduction of mesoporosity in zeolites using surfactants. The energy required for the hierarchization of USY zeolite by surfactant-templating is very similar to that involved in its crystallization. This suggests that the reorganization of the zeolite can be easily achieved. The kinetics of the whole process are highly dependent on the temperature. The results confirm that mesostructuring of USY zeolite with surfactants is energetically feasible under the conditions used in the synthesis of zeolites.
- The energetics of mesopore formation in zeolites with surfactants,
Noemi Linares, Erika de Oliveira Jardim, Alexander Sachse, Elena Serrano, Javier Garcia-Martinez,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201803759