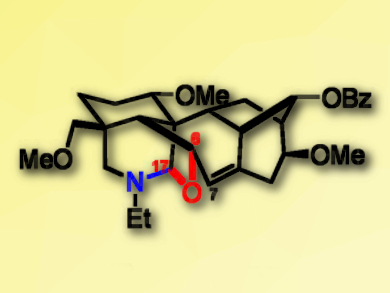
Franchetine (pictured), a unique 7,17-seco-type norditerpenoid alkaloid, shows a highly congested hexacyclic architecture containing a characteristic N,O-acetal moiety which distinguishes it from norditerpenoid alkaloids. Some derivatives of this compound have shown promising analgesic (pain-relieving) activity with little toxicity, indicating it may be used as a lead molecule to develop new analgesic agents.
Jian‐Li Wang, Liang Xu, and colleagues, Sichuan University, China, have developed an efficient synthesis of the hexacyclic framework of franchetine with the purpose of finding a new synthetic route to the natural molecule. The team started from a known tricyclic compound bearing a bicyclo[2.2.2]octane subunit. The six-membered A ring was formed through a radical cyclization of a iodoalkyne. The resulting exomethylene group was used to construct the C4 quaternary carbon of the target molecule. A reductive amination of the aldehyde installed the nitrogen atom, forming the N,O-acetal. Finally, the complete hexacycle of franchetine was formed by converting the bicyclo[2.2.2]octane moiety into a bicyclo[3.2.1]octane unit via a biomimetic Wagner–Meerwein rearrangement.
The synthesis involves 20 steps overall. According to the researchers, this method of constructing hexacyclic compounds could be an important methodological foundation for the synthesis of natural franchetine and related norditerpene alkaloids, such as aconitine.
- Expedient Construction of the Hexacycle of Franchetine,
Zhengchao Lv, Lingzhi Gao, Chuanxu Cheng, Wei Niu, Jian-Li Wang, Liang Xu,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800116