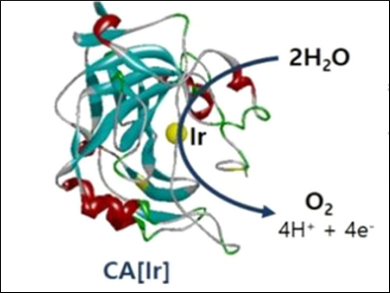
Water oxidation occurs in nature at the photosystem II, producing electrons and oxygen. Recently, water-oxidizing catalysts have attracted research attention as a key technology for renewable energy production.
A biological water-oxidation catalyst can be prepared by substituting the natural zinc cofactor of the enzyme carbonic anhydrase (CA) with iridium. Min-Chul Kim and Sang-Yup Lee, Yonsei University, Seoul, South Korea, have observed oxygen evolution in a water-oxidation process that was catalyzed by this Ir-substituted CA, abbreviated CA[Ir].
The team performed thermodynamic analyses on the Ir binding to the apo-CA protein (the protein without a co-factor). They found that coordination of Ir to the apoprotein of CA is thermodynamically preferred, which implies that the CA apoprotein is stabilized by Ir binding.
CA[Ir] showed comparable catalytic activity to other Ir-based chemical catalysts. The modified enzyme has a turnover frequency (TOF) of 39.8 min−1 under mild and neutral conditions (30 °C, pH 7). The biological character of CA[Ir] could be beneficial for the development of future eco-friendly, enzymatic catalysts that require good biocompatibility.
- Catalytic Water Oxidation by Iridium-Modified Carbonic Anhydrase,
Min-Chul Kim, Sang-Yup Lee,
Chem. Asian J. 2018, 13, 334–341.
https://doi.org/10.1002/asia.201701543