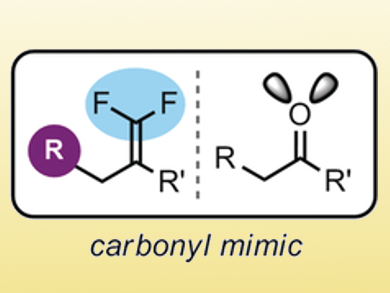
Gary A. Molander and colleagues, University of Pennsylvania, Philadelphia, USA, have developed a method for the synthesis of gem-difluoroalkenes. These fluorinated compounds could serve as ketone mimics in pharmaceutical agents. Ketones themselves are uncommon because of their propensity to undergo reduction by aldo-keto reductases.
The team used a photoredox-promoted radical/polar crossover reaction, which provides a convergent route to the 1,1-difluoroalkene motifs under mild conditions. Primary, secondary, and tertiary radicals bearing a wide range of functional groups are produced in photocatalytic reactions. These radicals can readily add to trifluoromethyl-substituted alkenes to provide the corresponding gem-difluoroalkenes (example pictured) in high yield.
The researchers have also developed a synthesis of a bench-top-stable organotrifluoroborate reagent for the facile synthesis of trifluoromethyl-substituted alkenes from aryl bromides. According to the team, this approach could enable medicinal chemists to efficiently synthesize a library of these biologically relevant molecules.
- Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics,
Simon B. Lang, Rebecca J. Wiles, Christopher B. Kelly, Gary A. Molander,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201709487