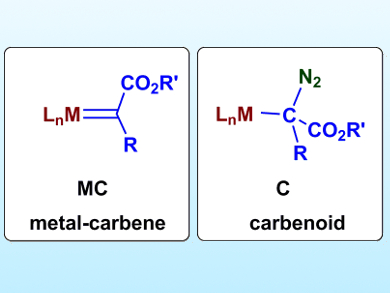
Carbenoid, a term first mentioned in the 1960s, has been used to refer to two different types of species (pictured), although this might not always be appropriate. A carbenoid according to IUPAC is a carbene-like species complexed to a metal that is or acts as a carbene.
The two types of species used for this term commonly appear in the Simmons-Smith reaction or in the metal-mediated carbene transfer from a diazo compound. Ana Caballero and Pedro Pérez, Universidad de Huelva, Spain, have explained the appropriate scenarios of when and when not to use the term carbenoid. The researchers describe metal-carbene complexes generated from diazo compounds as Fischer-type carbenes, not carbenoids. The interaction of a diazo compound with a metal center can lead to a carbenoid that is the precursor for a metal-carbene.
True carbenoids are defined here as compounds or formula R’R’’C(M)X with a central, tetrasubstituted carbon atom carrying a leaving group X and a metal-carbon bond. When X leaves, CR’R’’ is eliminated and can be transferred to another substrate. The team has cleared up some confusion that was apparent in the literature regarding carbenoid and metal-carbene terminology.
- Dimensioning the Term Carbenoid,
Ana Caballero, Pedro J. Pérez,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201702392