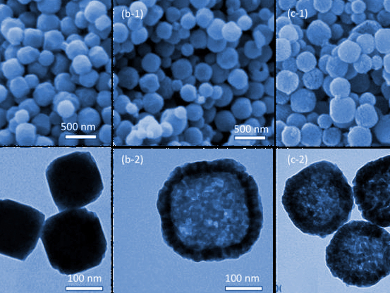
Iron pyrite or “Fool’s Gold” (FeS2) has drawn a lot of research attention due to its abundance and useful properties, such as high optical absorbance and non-toxicity. It has wide-ranging applications from heavy-metal absorption to batteries to solar cells. Fine control over its morphology, however, remains difficult due to complicated reaction conditions and numerous side products.
Kevin C.-W. Wu, Kuo‐Chuan Ho, National Taiwan University, and colleagues have developed a method to make hollow iron pyrite nanoparticles (pictured). The multi-step process starts with Prussian Blue. Although better known as a blue pigment, it is also a useful iron cyanide reagent. The team first etched the Prussian Blue with acid to form hollow particles, followed by an oxidation to remove the organic components, and finally sulfurization to create hollow particles of iron pyrite.
To demonstrate the properties of the hollow material, it was tested as a counter electrode in a dye-sensitized solar cell, and compared with both solid pyrite and a commercially used platinum counter-electrode material. Hollow pyrite particles gave a photocurrent conversion efficiency (η) of 7.31 %, which is far superior to the 5.30 % of the solid pyrite. The η value is close to that of platinum (7.69 %), which demonstrates the nanoparticles’ potential as a low-cost replacement for the expensive, scarce, precious metal.
- Metal-Organic Frameworks (MOFs)-Derived Synthesis of Hollow Porous Iron Pyrite Nanoparticles as Platinum-Free Counter Electrodes for Highly Efficient Dye-Sensitized Solar Cells,
Kevin Chia-Wen Wu, Jeffrey E. Chen, Miao-Syuan Fan, Yan-lin Chen, Yu-Heng Deng, Jung Ho Kim, Yusuke Yamauchi, Kuo-Chuan Ho,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201702687