The methanol-to-hydrocarbons (MTH) reaction promises to be a major source for fuel and olefins in a post-oil society. Consequently, the reaction is under close scrutiny and more than 20 “direct” mechanisms, whereby methanol/dimethyl ether molecules are combined to form ethene, have been proposed for the initial C–C bond-forming step alone.
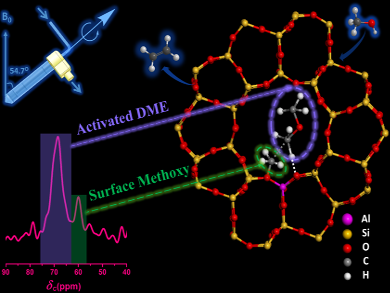
Zhongmin Liu, Yingxu Wei, and colleagues, Dalian Institute of Chemical Physics, China, have used in situ solid-state NMR spectroscopy to study methanol conversion over a zeolite catalyst (HZSM-5) at 300 ºC. Using solid-state cross-polarization magic angle spinning 13C NMR spectroscopy, the team detected a methyleneoxy analogue on the surface of HZSM-5, which originated from surface-activated dimethyl ether.
This finding provides evidence for a direct mechanism in the MTH reaction, where a C–H bond activation of adsorbed dimethyl ether or methanol by neighboring surface methoxy groups or trimethyloxonium cations is a prelude to the first C–C bond formation step of C1 reactants.
- Direct Mechanism of the First Carbon-Carbon Bond Formation in the Methanol-to-Hydrocarbons Process,
Xinqiang Wu, Shutao Xu, Wenna Zhang, Jindou Huang, Jinzhe Li, Bowen Yu, Yingxu Wei, Zhongmin Liu,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201703902