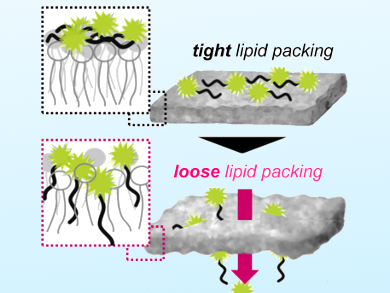
Arginine-rich cell-penetrating peptides (CPPs) can deliver membrane-impermeable bioactive molecules into cells under certain conditions. Hydrogen bonding and ion pairing between the guanidino groups of arginine-rich CPPs and lipid headgroups is important in membrane binding. However, the mechanism by which hydrophilic cationic CPPs are conveyed across the apolar membrane is still unclear.
Shiroh Futaki, Kyoto University, Japan, and colleagues have identified lipid packing loosening as a key factor in the direct translocation of octaarginine across HeLa cell membranes (pictured). Confocal laser scanning microscopy was used to detect the translocation of octaarginine labeled with a polarity-sensitive dye (di-4-ANEPPDHQ). A significant lipid packing loosening was observed in cell membranes treated with different curvature-inducing peptides.
The team suggests that the membrane distortion enhances hydrophobic interactions between the peptide backbone and exposed lipid acyl chains, thereby promoting the transport of CPPs across the cell membrane. The findings may be relevant to viral infection mechanisms, such as that involving HIV-1 tat—an arginine-rich regulatory protein that enhances the viral transcription of HIV DNA.
- Loosening of Lipid Packing Promotes Oligoarginine Entry into Cells,
Tomo Murayama, Toshihiro Masuda, Sergii Afonin, Kenichi Kawano, Tomoka Takatani-Nakase, Hiroki Ida, Yasufumi Takahashi, Takeshi Fukuma, Anne S. Ulrich, Shiroh Futaki,
Angew. Chem. Int. Ed. 2017, 56, 7644–7647.
DOI: 10.1002/anie.201703578