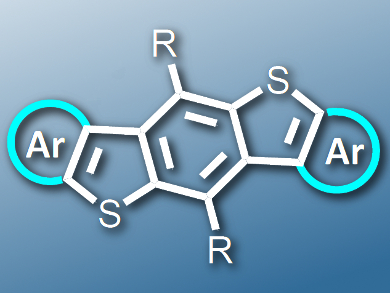
Linear heteroacenes, which contain linearly annulated heterocycles, are some of the most studied materials for organic electronic devices because of their unique electronic structures. In addition, the heteroatoms offer great potential to fine-tune the electronic structures of the materials.
Lei Zhang, Beijing University of Chemical Technology, China, and colleagues have developed a straightforward strategy for the construction of a class of heteroacenes by the lateral extension of benzodithiophene (BDT). The synthetic strategy relies on the intramolecular cyclization of a chalcogenol to an ethynyl group to give heteroacenes with five fused rings. These novel linear acenes contain thiophene, selenophene, and tellurophene as the outmost rings (example pictured).
Changing the heteroatomes in the outermost rings, allows to modulate the optical, electrochemical properties and crystal packing motifs of the acenes. The resulting heteroacenes are promising building blocks for the preparation of new donor–acceptor copolymers, as well as for even larger heteroacenes for organic electronic devices.
- Lateral Extension of Benzodithiophene System: Construction of Heteroacenes Containing Various Chalcogens,
Lei Zhang, Jichang Wei, Dong Meng, Zhaohui Wang,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700552