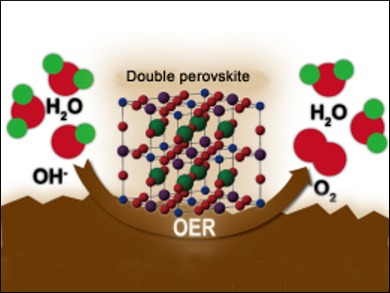
Highly efficient electrocatalysts for the oxygen evolution reaction (OER) play a crucial role in several energy storage and conversion systems, such as reversible fuel cells, rechargeable metal-air batteries, and water splitting. Noble metal oxides (e.g., IrO2 and RuO2) have been identified as the state-of-the-art catalysts to promote OER, but their high cost, scarcity, and poor long-term stability heavily hamper their widespread commercialization
Wei Zhou, Zongping Shao, Nanjing Tech University, China, have been investigating oxide materials as potential OER catalysts. Double perovskite oxides of the type ABB’O6 (A,B,B’ = metals) are an extremely versatile class of materials. Many combinations of metals can fill the A, B, and B’ sites, resulting in compounds that may have a wide range of interesting properties and uses from ferromagnetism and large magnetoresistance through to fuel cell electrodes and, in this case, catalysts.
oxide materials as potential OER catalysts. Double perovskite oxides of the type ABB’O6 (A,B,B’ = metals) are an extremely versatile class of materials. Many combinations of metals can fill the A, B, and B’ sites, resulting in compounds that may have a wide range of interesting properties and uses from ferromagnetism and large magnetoresistance through to fuel cell electrodes and, in this case, catalysts.
The best result for OER catalysis was achieved by Ba2Bi0.1Sc0.2Co1.7O6-δ with Ba on the A-site, Bi and Sc on the B’-site, and cobalt on both B and B’-sites. The splitting of cobalt across two sites is thought to be critical as it results in double the number of active centers for the catalytic reaction. This explains the increased performance compared to existing OER catalysts.
- B-Site Cation Ordered Double Perovskites as Efficient and Stable Electrocatalysts for Oxygen Evolution Reaction,
Hainan Sun, Gao Chen, Yinlong Zhu, Bo Liu, Wei Zhou, Zongping Shao,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201700507