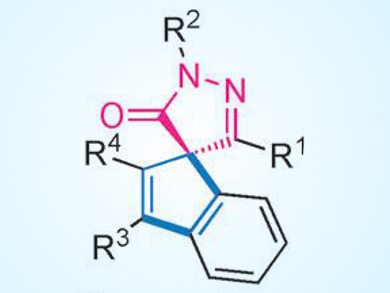
4-Spiro-5-pyrazolones are important molecular scaffolds that occur widely in natural products, biologically active molecules, and pharmaceutical agents. However, the preparation of these compounds in enantioenriched forms is challenging. Previously reported synthesis methods often use substrates that are not readily accessible.
Shu-Li You, Chinese Academy of Sciences, Shanghai, and Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, China, and colleagues have developed a rapid route to highly enantioenriched spiro[indene-1,4′-pyrazol]-5′(1′H)-one derivatives (example pictured). The approach involves a Rh-catalyzed C(sp2)−H functionalization/annulation strategy that starts from readily available pyrazolones and alkynes. The use of a rhodium catalyst derived from a chiral cyclopentadienyl ligand (based on the privileged 1,1’-spirobiindane scaffold) is crucial for obtaining excellent enantioselectivity (up to 98 % ee).
The team demonstrated the synthetic utility of this method by a straightforward gram-scale reaction and diverse transformations of the products without loss of the enantiopurity. The method advances the field of asymmetric C–H direct functionalization, and the novel spiropyrazolone products could be interesting for medicinal chemists.
- Asymmetric Synthesis of Spiropyrazolones by Rhodium-Catalyzed C(sp2)–H Functionalization/Annulation Reactions,
Jun Zheng, Shao-Bo Wang, Chao Zheng, Shu-Li You,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201700021