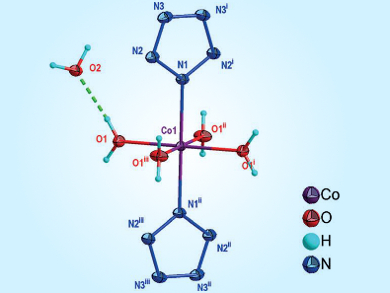
The synthesis of pentazole (N5) and trapping of the pentazolate anion with a metal ion have always been a challenge. Bingcheng Hu, Nanjing University of Science and Technology, China, Chengguo Sun, University of Science and Technology Liaoning, China, and colleagues have developed a simple and direct method for the synthesis of a stable pentazole-based metal species, Co(N5)2(H2O)4•4 H2O.
The synthetic process involves two steps: The pentazolate salt (N5)6(H3O)3(NH4)4Cl was obtained by a direct cleavage of the C–N bond in a multisubstituted arylpentazole using m-chloroperbenzoic acid and ferrous bisglycinate, and cyclo-N5– was subsequently captured from (N5)6(H3O)3(NH4)4Cl with Co2+ ions to form Co(N5)2(H2O)4•4 H2O. The structure was confirmed by Raman and infrared (IR) spectroscopy as well as single-crystal X-ray diffraction analysis.
The team showed that Co(N5)2(H2O)4•4 H2O had a higher energy density and produces stronger explosions than (N5)6(H3O)3(NH4)4Cl. This complex has a high percentage of nitrogen and has potential applications in environmentally friendly and high-performing materials for propellants and explosives.
- A Symmetric Co(N5)2(H2O)4·4 H2O High-Nitrogen Compound Formed by Cobalt(II) Cation Trapping of a Cyclo-N5– Anion,
Chong Zhang, Chen Yang, Bingcheng Hu, Chuanming Yu, Zhansheng Zheng, Chengguo Sun,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201701070