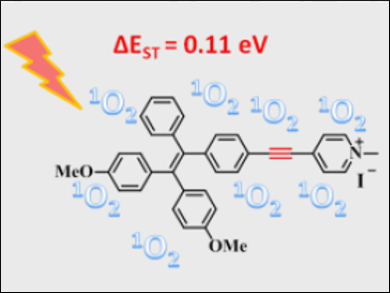
Conventional fluorophores usually suffer from concentration quenching. This leads to decreases in photosensitizing efficiency. However, for fluorophores with aggregation-induced emission (AIE) characteristics, the excited states become more active in the aggregated states. This makes AIE fluorogens potentially interesting as photosensitizers for biological applications.
Deqing Zhang, Guanxin Zhang, Chinese Academy of Sciences, Beijing, China, and colleagues have shown that the photosensitizing efficiency can be significantly enhanced by insertion of an alkyne group. A pyridinium-substituted tetraphenylethylene with an alkyne group (TPE-A-Py+, pictured) shows a higher photosensitizing efficiency than an analogue without an alkyne group. Iodide is used as the counteranion.
The researchers explained the efficiency by a decrease of the energy gap between the excited singlet state and triplet state upon insertion of the alkyne moiety. Moreover, TPE-A-Py+ can be used at low concentrations as an efficient photosensitizer to quickly photo-inactivate ampicillin-resistant Escherichia coli bacteria under white-light irradiation.
- Pyridinium substituted tetraphenylethylene entailing alkyne moiety: enhancement of photosensitizing efficiency and antimicrobial activity,
Xue You, Huili Ma, Yuancheng Wang, Guanxin Zhang, Qian Peng, Libing Liu, Shu Wang, Deqing Zhang,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700243