Porous coordination polymers (CP) are useful in microelectronics and smart membranes. Otherwise known as metal-organic frameworks or MOFs, the repeating units of CPs are coordination complexes. Preparation of such CPs can be done in solution; however, the inclusion of solvent molecules into the CP and their subsequent removal remain problematic. Vapor-phase methods can circumvent this challenge, but these methods have not been fully optimized to fabricate porous crystalline CPs.
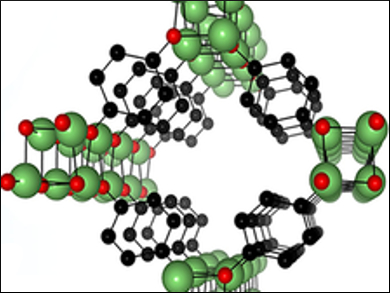
Maarit Karppinen and colleagues, Aalto University, Finland, have fabricated CPs composed of lithium and hydroquinone (Q) using a combination of gas-phase atomic and molecular layer deposition techniques. The films were deposited using lithium hexamethyldisilazide (LiHMDS) and hydroquinone as precursors at temperatures between 105 °C and 280 °C. The team studied the properties of the resulting lithium aryloxide CPs (Li2Q) using X-ray diffraction and IR spectroscopy, as well as density functional theory (DFT) calculations. Both experimental and theoretical results suggest the formation of porous crystalline CP thin films.
Notably, the team saw differences between air-exposed Li2Q and pristine Li2Q (protected by a coating of Al2O3). Air exposure led to a uniform change in color and thickness. The researchers attribut these differences to the coordination of water molecules to the Li centers in the Li2Q thin films. After heating the air-exposed samples to 120 °C and then coating with Al2O3, the resulting Li2Q films were similar to the pristine Li2Q. This shows that the fabricated Li2Q can accommodate guest molecules (in this case, water) uniformly throughout the thin film, although the reversibility of the absorption is not ideal. According to the researchers, the work could be the basis for the development of gas-phase layer-by-layer synthesis of CPs with advanced functionalities.
- Lithium Aryloxide Thin Films with Guest-Induced Structural Transformation by ALD/MLD,
Mikko Nisula, Jarno Linnera, Antti J. Karttunen, Maarit Karppinen,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201605816