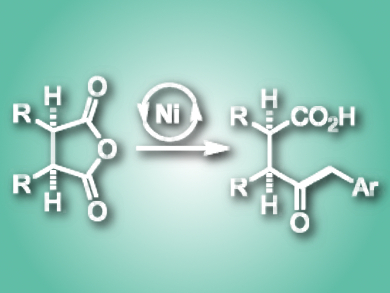
The success of light-driven catalysis to form challenging bonds has inspired the development of nickel/photoredox-catalyzed cross-coupling reactions. The use of this catalytic scheme in asymmetric transformations, however, has seen limited application.
Tomislav Rovis, Colorado State University, Fort Collins, USA, and Columbia University, New York, USA, Abigail G. Doyle, Princeton University, NJ, USA, and colleagues have developed a nickel-catalyzed desymmetrization of cyclic meso-anhydrides. The reaction uses benzylic radicals formed from photoredox-mediated oxidations of benzylic trifluoroborate salts. In the presence of Ni(cod)2, (–)-2,2′-isopropylidenebis-(4S)-4-phenyl-2-oxazoline (PhBox), benzyl trifluoroborate, and the inexpensive organic photocatalyst 2,4,5,6-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN), under irradiation by blue LEDs, cyclic meso-anhydrides were converted to the corresponding cis-keto-acid products. The reaction proceeds in high yield and with high enantioselectivity and diastereoselectivity.
The reaction is tolerant of numerous cyclic anhydrides, as well as electronically and sterically diverse trifluoroborates. Additionally, the researchers could access the enantioenriched trans product in modest selectivity via an epimerization event on the oxidative addition adduct by simply modifying the catalyst loading. This work represents only the second example of a highly enantioselective metallo-photoredox cross-coupling reaction.
- Dual Nickel- and Photoredox-Catalyzed Enantioselective Desymmetrization of Cyclic meso-Anhydrides,
Erin E. Stache, Tomislav Rovis, Abigail G. Doyle,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201700097