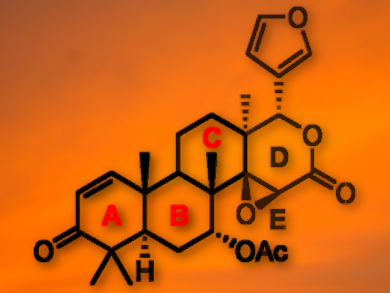
The naturally occurring tetranortriterpene gedunin (pictured) has a wide range of important biological properties (antimalarial, insecticidal, anti-cancer). Although this molecule has received substantial research attention, there has not been a de novo synthesis since it was first isolated in 1960.
Craig Williams, University of Queensland, St. Lucia, Australia, and colleagues have discovered the first synthetic route to the complete ABC ring system of gedunin. Key transformations in this sequence include: (i) consecutive stereoselective annulation reactions, (ii) a Pd-catalyzed epoxide reduction which relies on triethylamine for the generation of a reactive Pd-imino species, and (iii) the protection of a β-hydroxy ketone as a 3-alkoxy dioxane, which proved resistant to dissolving metal reduction, amide and hydride bases, and oxidation with H2O2, but was cleaved using mildly acidic conditions.
The researchers successfully synthesized the fully decorated ABC ring system of gedunin, incorporating the 7-α-acetoxy residue, which until now lacked a versatile protocol. These results could help to further develop medicinal chemistry based on this biologically important molecule.
- Towards the Total Synthesis of Gedunin: Construction of the Fully Elaborated ABC Ring System,
David M. Pinkerton, Paul V. Bernhardt, G. Paul Savage, Craig M. Williams,
Asian J. Org. Chem. 2017.
DOI: 10.1002/ajoc.201700013