Radical processes are one of the most efficient approaches for the transformation of olefins. However, the high reactivity of radical species means that the selective control of carbon radical intermediates is extremely challenging. Enantioselective radical reactions, which remained unexplored for a long time, are atrracting research interest.
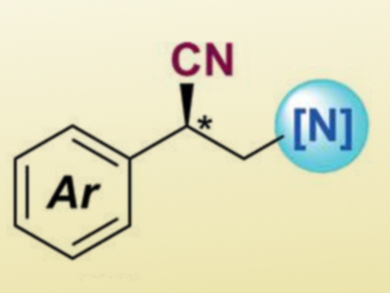
Zhenyang Lin, Hong Kong University of Science and Technology, China, Guosheng Liu, Chinese Academy of Sciences, Shanghai, and colleagues have discovered that a bisoxazoline/copper(II) cyanide complex can enantioselectively trap benzylic radicals to provide chiral alkylnitriles (example pictured). This is an efficient method to control the stereoselectivity of radical reactions.
The team performed enantioselective copper-catalyzed intermolecular amino- and azidocyanations of alkenes. Various β-amino nitrile derivatives were obtained with good to excellent enantioselectivity. Such chiral β-amino nitriles commonly exist in natural products and bioactive molecules. They could also be transformed to chiral 1,3-diamines, which are an important class of compounds in organic synthesis.
- Enantioselective Copper-Catalyzed Intermolecular Amino- and Azidocyanation of Alkenes in a Radical Process,
Dinghai Wang, Fei Wang, Pinghong Chen, Zhenyang Lin, Guosheng Liu,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201611850