Rechargeable batteries with organic electrodes are preferable to those with transition-metal-containing electrodes for their environmental friendliness and resource availability. However, all such batteries reported to date are based on organic electrolytes, which raise concerns over their safety and performance. Most organic electrodes cannot be used in aqueous electrolytes due to unfavorable side reactions.
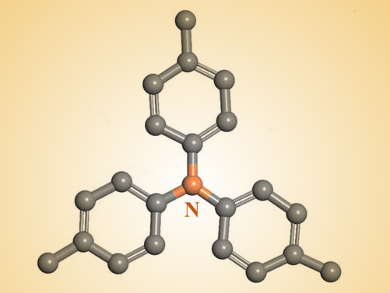
Donald G. Truhlar, University of Minnesota, Minneapolis, USA, Yonggang Wang, Fudan University, Shanghai, China, and colleagues have developed an all-organic battery with polytriphenylamine (structure partially pictured above) as the cathode material and a 1,4,5,8-naphthalenetetracarboxylic dianhydride-derived polyimide as the anode material.
In normal aqueous electrolytes, a side reaction occurs, forming oxygen and rendering the system irreversible. The team overcame this difficulty by using a “water-in-salt” electrolyte. This is made from a highly concentrated solution of lithium bis(trifluoromethanesulfonyl) imide. The high concentration prevents the side reactions that occur in normal aqueous electrolytes. This allows the battery to reach its full potential, with a high power and energy density (32000 W kg–1, 52.8 W h kg–1), high cycling stability, and low cost.
- All-Organic Rechargeable Battery with Reversibility Supported by “Water-in-Salt” Electrolyte,
Xiaoli Dong, Hongchuan Yu, Yuanyuan Ma, Junwei Lucas Bao, Donald G. Truhlar, Yonggang Wang, Yongyao Xia,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201700063