The Rh(I)-catalyzed C–C bond activation of vinylcyclopropanes (VCPs) is a well-explored strategy for building all-carbon rings. It has been proposed that the C–C bond cleavage occurred between C(1) and C(2) to form σ,η3-allyl rhodium species. However, direct experimental evidence was lacking.
Jianbo Wang and colleagues, Peking University, China, have reported the first example of a rhodium-carbene induced C–C bond cleavage of a C(2)–C(3) bond in siloxylvinylcyclopropanes. The team used [Rh(cod)Cl]2 (cod = 1,5-cyclooctadiene) to catalyze the reaction of siloxylvinylcyclopropanes with diazoesters. E-configured alkene products were obtained in good yields. The researchers assume that increasing the steric hindrance around the rhodium center, by forming rhodium carbene, leads to the C(2)–C(3) bond cleavage.
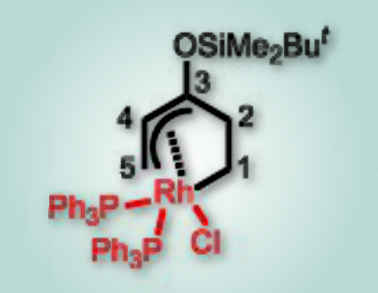
In addition, the researchers have isolated and obtained the single-crystal structure of a σ,η3-allyl rhodium species (pictured) from the stoichiometric reaction of the Rh(I) catalyst with siloxylvinylcyclopropane. They could show that it is the key intermediate of the Rh-catalyzed cycloaddition between siloxylvinylcyclopropane and alkynes.
- Rhodium(I)-Catalyzed C−C Bond Activation of Siloxyvinylcyclopropanes with Diazoesters,
Sheng Feng, Fanyang Mo, Ying Xia, Zhenxing Liu, Zhen Liu, Yan Zhang, Jianbo Wang,
Angew. Chem. Int. Ed. 2016, 55, 15401–15405.
DOI: 10.1002/anie.201609642