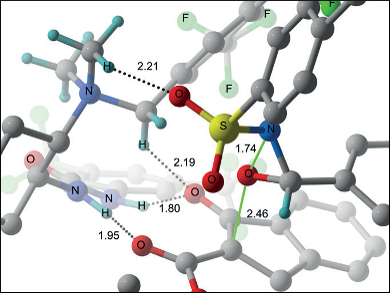
α-Hydroxy-β-dicarbonyl structures are often found in biologically active compounds such as pharmaceuticals. They can be synthesized through direct α-hydroxylation with either chiral auxiliaries or enantioselective catalysts.
Mario Waser, Johannes Kepler University, Linz, Austria, and colleagues, have used chiral urea-containing ammonium salts as bifunctional catalysts that not only catalyze the direct α-hydroxylation of β-ketoesters with oxaziridines under base-free conditions, but also enable the simultaneous kinetic resolution of these oxaziridines. Using this catalyst system, the researchers were able to obtain the desired α-hydroxy-β-dicarbonyl products in up to 97 % yield after two hours with enantiomeric ratios of up to 99:1. In addition to this, increasing the amount of oxaziridine in the reaction mixture resulted in significant enantioenrichment of the unreacted oxaziridine up to an enantiomeric ratio of 94:6.
The researchers also observed a pronounced dependence of the effectiveness of the reaction on the enantiomeric composition of catalyst and oxaziridine, with a considerably higher reactivity for the matched pairs (both S,S– or both R,R-configured). These bifunctional catalysts are a promising tool for asymmetric synthesis of biologically relevant compounds.
- Bifunctional Ammonium Salt Catalyzed Asymmetric α-Hydroxylation of β-Ketoesters by Simultaneous Resolution of Oxaziridines,
Johanna Novacek, Joseph A. Izzo, Mathew J. Vetticatt, Mario Waser,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201604153