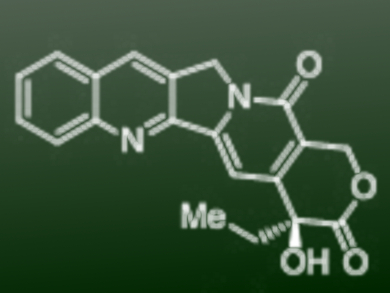
Camptothecin (CPT, pictured) is a pentacyclic quinoline alkaloid that was isolated from Camptotheca acuminata trees in 1966. Its potent anticancer activity has attracted attention from both the academic and pharmaceutical communities. The commercially available anticancer drugs topotecan (Hycamtin), belotecan, and irinotecan (Camptosar) are CPT analogues.
Shuanhu Gao and colleagues, East China Normal University, Shanghai, have developed a flexible strategy for the total synthesis of CPT and related natural alkaloids containing indolizinone or quinolizinone. The team devised a cascade reaction that occurs under mild conditions in good regioselectivity through an exo-type hydroamination, followed by a spontaneous lactamization. The reaction precursors can be efficiently prepared from easily available building blocks through a convergent manner using a Sonogashira coupling reaction. This approach was used in the total synthesis of camptothecin in nine steps.
The same method was used to synthesize five natural alkaloids which are structurally related to CPT, namely 22-hydroxyacuminatine, oxypalmatine, norketoyobyrine, naucleficine, and nauclefine, as well as 35 other analogues. According to the researchers, this synthetic approach – and the small-molecule library prepared with it – can open new avenues for studying the bioactivity of camptothecin-like natural products.
- Total Synthesis of Camptothecin and Related Natural Products by a Flexible Strategy,
Ke Li, Jinjie Ou, Shuanhu Gao,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201607832