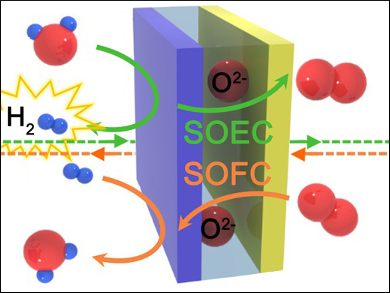
The electrolysis of water has attracted a great deal of interest for the clean production of hydrogen and as a highly efficient energy-storage system. In particular, high-temperature solid oxide electrolysis cells (SOECs) have received considerable attention because of their high efficiency. However, SOECs that use conventional electrodes suffer from performance degradation owing to delamination, poor redox stability, and coarsening of the electrodes during electrolysis.
Guntae Kim, Ulsan National Institute of Science and Technology (UNIST), Republic of Korea, and colleagues have developed highly efficient SOECs which can be used in continuous hydrogen production for more than 600 hours. The researchers used layered perovskites, PrBaMn2O5+δ (PBM) and PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (PBSCF50), as both electrodes. These are intrinsically stable under electrolysis operating conditions.
The researchers achieved a current density of 1.31 A cm–2 for the SOEC using PBM and PBSCF50 as electrodes at 800 °C and 1.3 V. They point out that this SOEC composed of layered perovskites is a very promising electrolytic system with high performance and stability.
- Achieving High Efficiency and Eliminating Degradation in Solid Oxide Electrochemical Cells Using High Oxygen-Capacity Perovskite,
Areum Jun, Junyoung Kim, Jeeyoung Shin, Guntae Kim,
Angew. Chem. Int. Ed. 2016, 55, 12512–12515.
DOI: 10.1002/anie.201606972