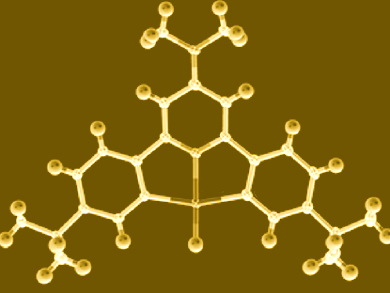
Gold catalysts have become increasingly important in organic transformations, yet their organometallic chemistry and reaction mechanisms are much less explored than those of other transition metals. The only known gold(III) hydrides are compounds in which all coordination sites are occupied by tightly bonded pincer ligands (example pictured). Without the ability to bind unsaturated substrates, it was therefore not obvious how such gold hydrides could possibly react to give insertion products.
Peter H. M. Budzelaar, University of Manitoba, Winnipeg, Canada, Manfred Bochmann, University of East Anglia, Norwich, UK, and colleagues have discovered that the reaction is promoted by traces of radicals. The addition of radical precursors reduces the reaction time from days to minutes. Small concentrations of odd-electron gold(II) species are produced which capture the substrate, followed by hydrogen transfer from another gold hydride molecule. This outer-sphere mechanism overcomes the limitations imposed by coordinative saturation and is supported by computational modeling. The reaction proceeds with almost complete regio- and stereoselectivity.
Since gold hydrides tolerate a large range of functional groups including carboxylic acids, this alkyne hydrometallation reaction can be extended to a wide range of substrates.
- Stereo- and Regioselective Alkyne Hydrometallation with Gold(III) Hydrides,
Anna Pintus, Luca Rocchigiani, Julio Fernandez-Cestau, Peter H. M. Budzelaar, Manfred Bochmann,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201607522