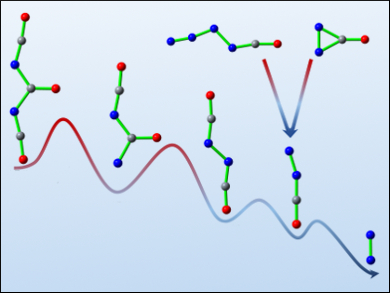
Small molecules solely consisting of N, C, and O have been the targets of extensive theoretical and experimental studies because of their fundamental importance in chemistry. As one of the simplest species, diazomethanone (NNCO) is formally a mixed dimer of N2 and CO. Its synthesis and characterization is challenging due to its metastability.
Xiaoqing Zeng, Soochow University, China, and colleagues have succeeded in generating this transient species and unraveling the formation mechanism using both experiment and theory. The team approached NNCO through the photolysis of carbonyl diisocyanate (OC(NCO)2) in a cryogenic argon matrix at 16 K. The stepwise decomposition of OC(NCO)2 into three CO molecules and one N2 molecule via isocyanatocarbonyl nitrene (OCNC(O)N), diisocyanate (OCNNCO), and NNCO (pictured) has been observed by matrix-isolation IR spectroscopy.
The formation of NNCO in the decomposition of the high-energy isocyanatoazide (OCNNNN) and diazirinone (cyclo-N2CO) has also been experimentally established. The isocyanatocarbonyl nitrene is the missing link in the previously studied Curtius rearrangement of N3C(O)NCO into OCNNCO.
- Photolysis of carbonyl diisocyanate: Generation of isocyanatocarbonyl nitrene and diazomethanone,
Qifan Liu, Hongmin Li, Zhuang Wu, Dingqing Li, Helmut Beckers, Guntram Rauhut, Xiaoqing Zeng,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201601073