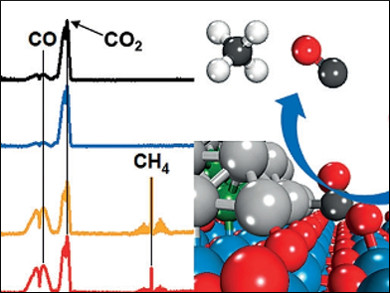
Chemical recycling of carbon dioxide to fuels, such as carbon monoxide, methane, or methanol, reduces atmospheric CO2 emission, which will be capped with quota restrictions in the future, and also generates useful products. The product selectivity, which strongly depends on the design of catalysts, has long been one of the major challenges for industrial applications.
Ping Liu, Jingguang Chen, Brookhaven National Laboratory, Upton, NY, USA, and colleagues found that the product selectivity in CO2 hydrogenation with a platinum-cobalt (PtCo) bimetallic catalyst can be controlled by simply changing the oxide support of the catalyst. A PtCo catalyst on a titanium dioxide support selectively converts CO2 to CO, which can be used as a feedstock in the Fischer-Tropsch process, an industrial process to produce chemicals and synthetic fuels from syngas (CO + H2). Using zirconium dioxide as a support selectively converts CO2 to CH4.
This study illustrates the feasibility of controlling the product selectivity in CO2 hydrogenation by fine-tuning the binding energies of the key reaction intermediates.
- CO2 Hydrogenation over Oxide-Supported PtCo Catalysts: The Role of the Oxide Support in Determining the Product Selectivity,
Shyam Kattel, Weiting Yu, Xiaofang Yang, Binhang Yan, Yanqiang Huang, Weiming Wan, Ping Liu, Jingguang G. Chen,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201601661