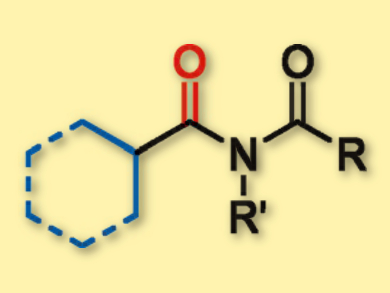
Carbonylation reactions are a useful method for the synthesis of carbonyl-containing compounds. However, the approach still faces some challenges, namely achieving direct C–H activation of the substrates, replacing expensive catalysts such as palladium with cheaper metals, e.g., copper, and applying the reactions to weak nucleophiles.
Xiao-Feng Wu and colleagues, Leibniz Institute for Catalysis at the University of Rostock, Germany, have developed a copper-catalyzed carbonylative C–H activation of cycloalkanes. With amides as the reaction partners and copper as the catalyst, the C(sp3)–H bonds of simple cycloalkanes were transformed into the corresponding imides (pictured) in good yields.
Since this reaction is an oxidative carbonylation reaction, air or other green oxidants can be used. According to the researchers, the use of copper catalysis is attractive for industrial applications.
- Copper-Catalyzed Carbonylative Coupling of Cycloalkanes and Amides,
Yahui Li, Kaiwu Dong, Fengxiang Zhu, Zechao Wang, Xiao-Feng Wu,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201603235