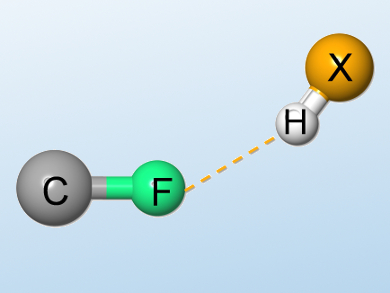
Strong hydrogen-bonding interactions play an important role in many chemical and biological systems. However, weak or very weak hydrogen bonds, which are often difficult to detect and characterize, may also be relevant in many recognition and reaction processes. The possibility of fluorine serving as a hydrogen-bond acceptor has been the subject of many controversial discussions.
Using established NMR techniques, Anna Vulpetti, Novartis Institutes for Biomedical Research, Basel, and Claudio Dalvit, University of Neuchâtel, both Switzerland, previously characterized and measured the strengths of intermolecular hydrogen-bond complexes involving the fluorine moieties CH2F, CHF2, and CF3, and compared them with the hydrogen-bond complex formed between acetophenone and the strong hydrogen-bond donor p-fluorophenol.
The team has found evidence for the formation of hydrogen bonds involving fluorine even with significantly weaker donors, namely 5-fluoroindole and water. The researchers used simple 19F NMR and 1H NMR titrations for the measurement of the strengths of hydrogen bonds. According to the researchers, such weak hydrogen bonds may be formed in the presence of high concentrations of hydrogen-bond donors and in the absence of strong hydrogen-bond acceptors, as well as in protein pockets shielded from the solvent. These results could have important implications for enzymatic and chemical reactions involving fluorine, and for ligand/protein 19F NMR screening.
- Weak Intermolecular Hydrogen Bonds with Fluorine: Detection and Implications for Enzymatic/Chemical Reactions, Chemical Properties, and Ligand/Protein Fluorine NMR Screening,
Claudio Dalvit, Anna Vulpetti,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201600446