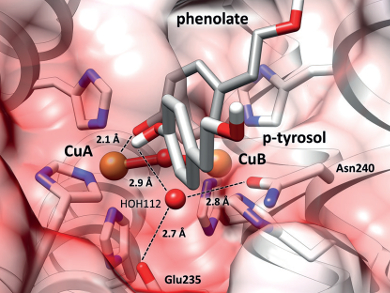
Tyrosinase enzymes catalyze the conversion of monophenols to ortho-quinones, while catechol oxidases only catalyze the oxidation of ortho-diphenols, leading to the same product. The reason for this difference in reactivity has been unclear, although it has been postulated that a glutamate and an asparagine unit in tyrosinases activate a water molecule, which then allows the conversion of monophenols to ortho-quinones.
Heinz Decker and Even Solem, Johannes Gutenberg University, Mainz, Germany, and Felix Tuczek, Albrechts University, Kiel, Germany, have found experimental evidence for this hypothesis. They have shown that a catechol oxidase, which converts only ortho-diphenols, can be turned into a tyrosinase by a mutation that introduces an asparagine residue.
The team produced a recombinant polyphenoloxidase from wine leaves which only catalyzes the oxidation of ortho-diphenols. They replaced a glycine with an asparagine by site-directed mutagenesis, and subsequently observed activity of the resulting enzymes in the conversion of monophenols to ortho-quinones
The tyrosinase activity thus depends on the presence of an asparagine, which along with a glutamate activates a conserved water towards deprotonation of monophenols. The reseachers hope that this improved understanding of the reactivities of these phenoloxidases will ease their modification, inhibition, and application in biotechnology.
- Tyrosinase versus Catechol Oxidase: One Asparagine Makes the Difference,
Even Solem, Felix Tuczek, Heinz Decker,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201508534