Nanozymes – enzyme mimetics based on nanomaterials – have potential applications in imaging, diagnostics, and therapeutics, although tuning their activity for specific applications by tailoring their chemical and physical properties remains a challenge.
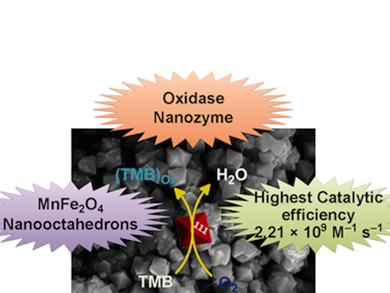
Govindasamy Mugesh and co-workers at the Indian Institute of Science, Bangalore, used a simple co-precipitation method in the presence of base to synthesize a MnFe2O4 nanomaterial. Remarkably, SEM and TEM analysis revealed a nanooctahedron (NOh) morphology and an average nanocrystal size of 150 nm.
The MnFe2O4 NOh was found to functionally mimic oxidase enzymes, successfully catalyzing the oxidation of several organic substrates in the presence of molecular oxygen. In particular, 3,3ʹ,5,5ʹ-tetramethylbenzidine was oxidized with a turnover number (kcat) and catalytic efficiency (kcat/KM) of 8.34×104 s−1 and 2.21×109 M−1s−1, respectively, which are the highest values for any nanozyme reported to date. Additional studies were performed using a range of techniques to understand the time-dependent evolution of this unexpected morphology and elucidate the catalytic mechanism.
- A Remarkably Efficient MnFe2O4-based Oxidase Nanozyme,
Amit A. Vernekar, Tandrila Das, Sourav Ghosh, Govindasamy Mugesh,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201500942
A MnFe2O4 nanooctahedron morphology was synthesized by a co-precipitation method and reported to be the most active nanozyme to date (kcat = 8.34×104 s−1, kcat/KM = 2.21×109 M−1s−1).

Find more world records from all branches of chemistry on the Records and Challenges platform of The Chemical Record.