Synergetic effects in bimetallic nanocatalysts are of great importance for improving the catalytic activity for various reactions. Due to the ill-defined interfacial structure of many bimetallic nanoparticles, it still remains challenging to understand these synergetic effects in depth.
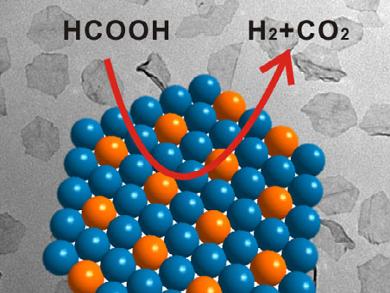
Nanfeng Zheng, Xiamen University, China, and colleagues have demonstrated that two-dimensional bimetallic materials are an ideal system to study such synergetic effects at the atomic level. By chemically depositing different amounts of Ag on ultrathin Pd nanosheets, they showed how the incorporation of Ag significantly enhances the catalytic properties for the selective decomposition of formic acid to produce H2 (pictured).
The superior activity is attributed to both electronic and geometric reasons. In addition to the electronic promotion of palladium by silver, the Pd-Ag interfaces created a geometric effect which prevents CO poisoning of the catalytic sites. In the future, using 2D materials as model catalysts may also shed light on synergetic effects in other reactions.
- Interfacial Effects in PdAg Bimetallic Nanosheets for Selective Dehydrogenation of Formic Acid,
Chengyi Hu, Xiaoliang Mu, Jingmin Fan, Haibin Ma, Xiaojing Zhao, Guangxu Chen, Zhiyou Zhou, Nanfeng Zheng,
ChemNanoMat 2015.
DOI: 10.1002/cnma.201500162