Genetic approaches are often used to identify potential target molecules to treat a given disease, usually proteins. Vast compound libraries are then screened for candidate lead molecules, and subsequent optimization efforts eventually result in the final drug. This process requires a tremendous amount of effort: identifying tractable pharmaceutical drug targets is difficult, and screening and optimization campaigns take time.
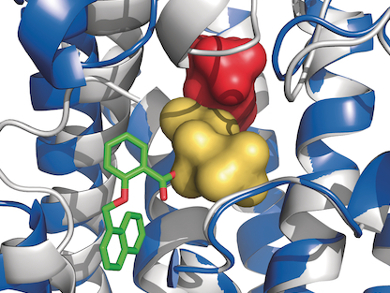
However, target identification can also occur in the reverse direction, starting from known drugs: Bisphosphonates are bone-targeting compounds that have been used for decades to treat osteoporosis and bone metastases. Their molecular target is an enzyme known as farnesyl pyrophosphate synthase (FPPS), which is essential for lipid production.
Recent evidence shows that bisphosphonates could also be effective in treating other conditions caused by excessive lipid production; these include a wide range of diseases such as breast cancer, multiple myeloma, Alzheimer’s disease, and premature ageing. Because bisphosphonates bind to bone very tightly, however, they cannot be used to treat these non-bone diseases.
In a collaborative effort, researchers at the Novartis Institutes for Biomedical Research led by Wolfgang Jahnke and Andreas Marzinzik, discovered new FPPS inhibitors. These compounds differ significantly from bisphosphonates, and do not bind to bone. Yet they do inhibit FPPS with high potency, similar to the clinically used bisphosphonates. After optimization, these novel inhibitors could then be developed to evaluate their potential for treating various other diseases caused by excessive lipid production.
- Discovery of Novel Allosteric Non-Bisphosphonate Inhibitors of Farnesyl Pyrophosphate Synthase by Integrated Lead Finding,
Andreas L. Marzinzik, René Amstutz, Guido Bold, Emmanuelle Bourgier, Simona Cotesta, J. Fraser Glickman, Marjo Götte, Christelle Henry, Sylvie Lehmann, J. Constanze D. Hartwieg, Silvio Ofner, Xavier Pellé, Thomas P. Roddy, Jean-Michel Rondeau, Frédéric Stauffer, Steven J. Stout, Armin Widmer, Johann Zimmermann, Thomas Zoller, Wolfgang Jahnke,
ChemMedChem 2015.
DOI: 10.1002/cmdc.201500338