Visible-light photoredox catalysis has been used to initiate organic transformations since the 1970s. However, only recently have spectacular advancements been made in this area. Owing to its infinitely available source, simple operation, and fascinating potential of applications, visible-light photoredox catalysis is emerging as a powerful tool in synthetic organic chemistry.
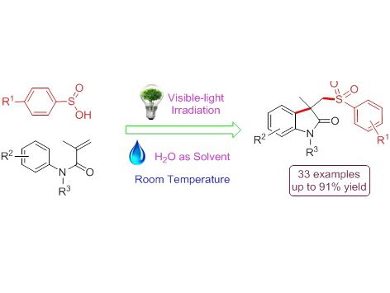
Lei Wang, Huaibei Normal University, China, and colleagues developed a visible-light photocatalytic difunctionalization of aryl acrylamides with aryl sulfinic acids to yield important sulfonated oxindoles via cascade C–S/C–C bond formation in water. Oxindoles are an important class of N-heterocycles with unique biological activity and are a privileged scaffold for drug discovery and library design. They are traditionally prepared by transition-metal-catalyzed cyclization or radical-initiated processes. This new methodology provides mild, efficient, and environmental friendly access to functionalized oxindoles in good to excellent yields, avoiding the use of metal catalysts, organic solvent, and high reaction temperature.
- Visible-Light Photoredox Catalysis: Direct Synthesis Sulfonated Oxindoles from N-Arylacrylamides and Arylsulfinic Acids via Cascade C-S/C-C Formation Process,
Lei Wang, Tao Miao, Pinhua Li, Dong Xia,
Chem. Asian J. 2015.
DOI: 10.1002/asia.201500498