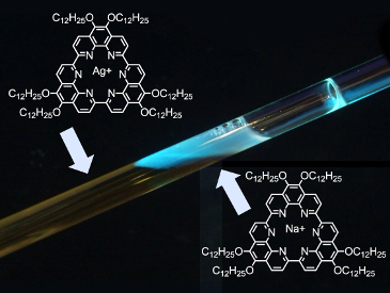
Nitrogen-containing macrocycles are an important class of compounds in organic chemistry and materials science. Klaus Müllen, Max Planck Institute for Polymer Research, Mainz, Germany, and colleagues in Belgium, Germany, and Japan have managed to synthesize a series of new toroidal macrocycles with high structural rigidity through the Yamamoto cyclotrimerization of functionalized 1,10-phenathrolines.
The researchers found that a number of alkaline-, transition-, and heavy-metal cations could be included as guests in the unusual hexaaza cavity of these cyclic ligands. The inclusion was accompanied by pronounced changes in the spectroscopic signature of the macrocycle. This property makes the ligand a useful candidate for sensing applications. The disc-shaped molecules also exhibit liquid-crystalline behavior, and the metal complexation ability could be exploited to build artificial molecular channels suitable for the transport of electrically charged guest species. In addition, well-ordered monolayers were observed at the solid/liquid interface through scanning tunneling microscopy (STM).
Further research is underway to reduce the metal ions sequestered within the cavities of the macrocycle to their neutral state under STM control. If this can be achieved, it is expected that this transformation will be accompanied by a strong increase in the STM contrast compared with their ionic state. Under such circumstances the STM tip could be imagined to be a “pencil”, selectively addressing individual molecules, which could lead to to “binary writing”.
- Torands Revisited: Metal Sequestration and Self-Assembly of Cyclo-2,9-tris-1,10-phenanthroline Hexaaza Macrocycles,
Matthias Georg Schwab, Masayoshi Takase, Alexey Mavrinsky, Wojciech Pisula, Xinliang Feng, José A. Gámez, Walter Thiel, Kunal S. Mali, Steven de Feyter, Klaus Müllen,
Chem. Eur. J. 2015.
DOI: 10.1002/chem.201406602