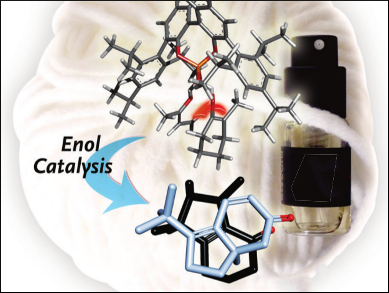
Design and Enantioselective Synthesis of new Cashmeran Odorants
When a perfume is said to include Cashmere Wood, it means the typical smell of the odorant Cashmeran. As described in the journal Angewandte Chemie, scientists from Switzerland and Germany have introduced new members in this exclusive and precious family of scents, by using a novel synthetic strategy to synthesize enantiomerically pure products. Olfactory analysis of these compounds provided insight into the structural requirements for Cashmeran odorants.
“In perfumes, Cashmeran is a particularly valuable perfumistic synthetic odorant because it combines conifer-type woody aspects perfectly with a musky odor, which is essential for the composition of trendy agarwood/oudh scents” reports Philip Kraft, Givaudan Schweiz AG, Dübendorf, Switzerland. High proportions of about 25 % Cashmeran, however, are also found in the perfumes “Dans Tes Bras” and “Duro”. Recently, this odorant has become increasingly popular in the perfumer’s palette. Cashmeran is an unsaturated ketone with a bicyclic framework made from one five- and one six-membered ring. Until now, it has been the only odorant in use with this special odor profile. Now, researchers working with Philip Kraft and Benjamin List, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Gemany, have made accessible a new class of enantiomerically pure Cashmeran odorants by using an new catalytic strategy.
Catalytic Enol Activation
The starting point was a weak open-chain with a high odor threshold, yet showing a certain Cashmeran character. To elaborate this feature, the researchers used computer simulations to superpose the lead structure onto that of Cashmeran and used the result to develop two target structures. In this reaction, one of the carbon centers is converted to a new stereocenter, which means that two compounds are possible which behave like an image and mirror image of each other. These two so-called enantiomeric forms can cause characteristic differences when binding to olfactory receptors.
“In order to obtain all four of the desired compounds, we had to develop a totally new synthetic strategy,” says List. “This is based on a novel Michael addition that occurs by means of a catalytic enol activation and opens exciting new prospects for the organic synthesis of enantiomerically pure substances.”
Characterizing the New Odor Family
While one of the compounds they produced shares the fruity aspects of Cashmeran, its enantiomer surprisingly brings the dark, woody facets of Cashmeran. Only the third of the new odorants combines both of them and displays a typical woody-musty Cashmeran odor. Unfortunately, in a direct comparison to Cashmeran, it turned out to be weaker. Compared to the lead structure, however, the activity is increased by 40 % – with increased Cashmeran character.
By comparing the odor characteristics of the four compounds, the researchers were able to figure out which configurations are crucial to the Cashmeran odor profile in this structurally new odor family – which allowed them to reach some preliminary conclusions with regard to the geometry of the olfactory receptors involved. Based on the different facets of Cashmeran displayed by the two enantiomers, the researchers concluded that at least two olfactory receptors are involved in the perception of Cashmeran odorants, though there are probably more.
- Design and Enantioselective Synthesis of Cashmeran Odorants by Using “Enol Catalysis”,
Irene Felker, Gabriele Pupo, Philip Kraft, Benjamin List,
Angew. Chem. Int. Ed. 2015.
DOI: 10.1002/anie.201409591