Magnetic Materials
We are all familiar with ordinary magnets in the form of metallic rods, horseshoes, or bars. However, magnets do not necessarily have to be made of metals or metal oxides. Researchers have also been exploring molecular magnets made from purely organic compounds, or by combining organic components with metal ions. In the journal Angewandte Chemie, Japanese researchers have now introduced a three-dimensional magnetic framework that is made from a combination of layer and pillar magnetic systems.
The advantage of molecular magnets is that they have properties similar to those of plastic instead of metal. Researchers are hoping for a class of materials with tailored optical, electrical, mechanical properties, as well as biocompatibility and other combinations of characteristics for use in applications for which conventional magnets are not suitable. These include electronic components like new storage media, as well as diagnostic and medical technology. However, this technology is still in its infancy. One of the practical problems is that most molecular magnets have so far only operated at extremely low temperatures.
In order for a material to become magnetic, there must be a macroscopically ordered arrangement of the individual “molecular magnets” (which are based on electron spins). Within a molecular framework, these are spaced too far apart to interact directly. However, certain constellations of chemical bonds can transmit the magnetic interactions. In systems made from different magnetic building blocks it is not easy to control the magnetic interactions between individual layers or chains.
Magnetic Layers and Chains Combine to form 3D Magnetic Framework
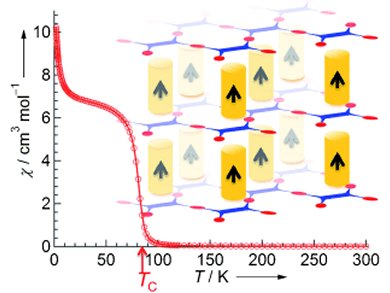
Hiroki Fukunaga and Hitoshi Miyasaka at Tohoku University, Japan, have now produced a three-dimensional structure with long-range magnetic order by combining a two-dimensional layer magnet and a one-dimensional columnar magnetic system. In the resulting pillared layer framework, the pillars determine the interactions between individual layers, enabling spontaneous magnetization.
The magnetic layer consists of paddlewheel-shaped complexes with an axis consisting of two ruthenium ions that are bound to four flat, organic “paddles”. The “paddlewheels” are bound into continuous layers by means of flat, organic semiconductor (tetracyanoquinodimethane, TCNQ). “Pillars” connect the layers into a three-dimensional lattice. The pillars are made of two flat molecules with an iron ion in-between. Charge-transfer and other electronic interactions take place between the two individual components. These allow the long-range magnetic order within the framework.
The material is magnetic below –191 °C (–166 °C under pressure). This sounds fairly cold, but is actually a pretty high temperature for a molecular magnet, which the researchers take as a good sign.
- Magnet Design by Integration of Layer and Chain Magnetic Systems in a π-Stacked Pillared Layer Framework,
Hiroki Fukunaga, Hitoshi Miyasaka,
Angew. Chem. Int. Ed. 2014.
DOI: 10.1002/anie.201410057